
Cobalt
Doublet Separations
- Co 2p: 14.99 eV
- Co 3p: 1.1 eV
The Energies Listed are Binding Energies!
- Co 2p: 780 eV
- Co 2s: 927 eV
- Co 3s 101 eV
- Co 3p: 60 eV
The Energies Listed are Binding Energies!
Co is primarily analyzed via the 2p orbital
- U 4d3/2 (781 eV)
- Ba 3d5/2 (781 eV)
- Fe LMM (Al source) (786 eV)
- Pr MNN (Al source) (788 eV)
- Ba 3d3/2 (796 eV)
- Hg 4s (800 eV)
Energies listed are Kinetic Energies!
Co LMM: ~ 780 eV
The Energies Listed are Binding Energies!
| Species | EB / eV | Charge ref | Ref |
| Co metal | 778.1 | Au 4f (83.95 eV) | 3 |
| CoO | 780 | C 1s (284.8 eV) | 1 |
| Co2O3 | 779.6 | C 1s (284.8 eV) | 1 |
| Co(OH)2 | 780.4 | C 1s (284.8 eV) | 1 |
| CoOOH | 780.1 | C 1s (284.8 eV) | 1, 6 |
| CoP | 778.3 | Au 4f (83.95 eV) | 3 |
Table 1: Typical biding energies for various common Co species
Analysis of Cobalt by XPS is typically performed on the intense 2p region, however as with most first row TMs, significant multiplet splitting renders deconvolution of Co complexes and compounds difficult (Figure 1). Co(II) and high spin Co(III) contain unpaired d-electrons and therefore the resultant spectra of these metal cations will possess complex structure.(1)
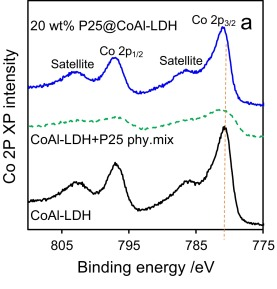
The only photoemission which is likely to cause issues with overlapping is Ba 3d, however Fe LMM, Pr MNN and even Co LMM augers (Al ka X-rays – 1486.7 eV) may overlap, though the Co LMM auger is only a minor overlap.
Co 2p peaks are separated by a sizable doublet separation (14.99 eV).
Work from Grosvenor et al determined that cobalt metal consists of one asymmetric photoemission and 2 plasmons (surface and bulk) at +3 and +5 eV above the photoemission (Figure 2).(2)
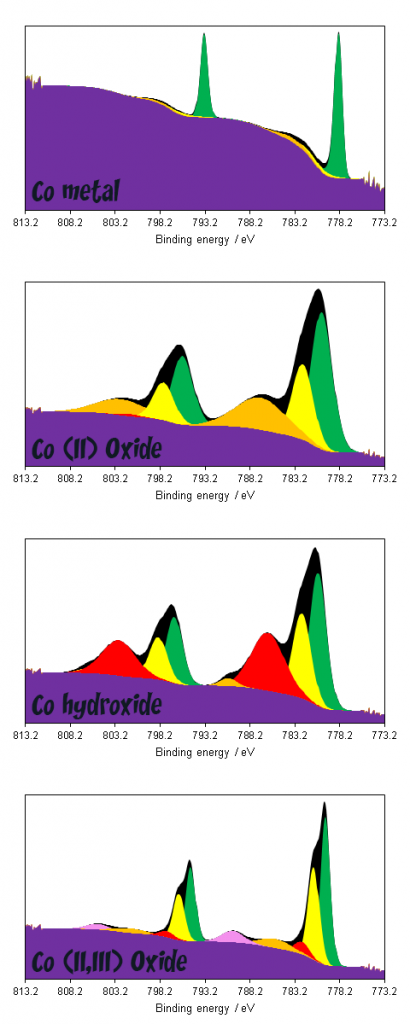
Cobalt compounds displaying multiplet splitting pose a much steeper challenge as far as data interpretation is concerned. As can be seen in figure 3, broad combinations of overlapping features render deconvolution of multiple compounds exceptionally challenging, whilst even deconvolution of metal and oxide/hydroxide requires non-standard modifications to the application of a background due to overlaps between metal 2p1/2 and oxide 2p3/2 satellites.(1,4)

The unique lineshapes do afford the possibility of fingerprint analysis however, either through utilising the theoretically derived fits of Biesinger(1) or using reference spectra of standard materials.
| Species | EB / eV | Charge ref | Ref |
| Co metal | 778.1 | Au 4f (83.95 eV) | 3 |
| CoO | 780 | C 1s (284.8 eV) | 1 |
| Co2O3 | 779.6 | C 1s (284.8 eV) | 1 |
| Co(OH)2 | 780.4 | C 1s (284.8 eV) | 1 |
| CoOOH | 780.1 | C 1s (284.8 eV) | 1, 6 |
| CoP | 778.3 | Au 4f (83.95 eV) | 3 |
Cobalt oxides reduced by Ar+ sputtering, use low energy for profiling or cleaning.
Cobalt 3s spectra, whilst relatively weak (and overlapping with some notable emissions such as Si 2p and Ce 4d) may also offer unique insights into the cobalt speciation since 3s region splitting (figure 4) of TMs may be sensitive to the metal spin state and ligand-metal charge transfer.(7)

Spin-induced Co 3s multiplet splittings for some common species may be found in table 2. Note that the splitting may not always be strongly observable, for example in Co metal it is present as a shoulder and often recorded as no splitting. Cobalt (III) oxide, as with the 2p region, exhibit negligible multiplet splitting compared with Co (II) species.
| Species | ΔE3s / eV | Ref |
| Co metal | 3.3+0.3 | 8 |
| CoF2 | 5 | 9 |
| CoF3 | 6 | 9 |
| CoO | 4.7 | 10 |
| Co3O4 | No splitting | 10 |
| Co(OH)2 | 5 | 10 |
Not available
Not available
- Biesinger, M. C., et al. (2011). “Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni.” Applied Surface Science 257(7): 2717-2730. Read it online here.
- Kumar, S., et al. (2017). “P25@ CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction.” Applied Catalysis B: Environmental 209: 394-404. Read it online here.
- Grosvenor, A. P., et al. (2005). “Examination of the Bonding in Binary Transition-Metal Monophosphides MP (M = Cr, Mn, Fe, Co) by X-Ray Photoelectron Spectroscopy.” Inorganic chemistry 44(24): 8988-8998. Read it online here.
- Biesinger, M. C., et al. (2009). “X‐ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems.” Surface and Interface Analysis. 41(4): 324-332. Read it online here.
- Spectra obtained and fit by HarwellXPS staff
- Yang, J., et al. (2010). “Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs.” The Journal of Physical Chemistry C 114(1): 111-119. Read it online here.
- Sarnecki, A., et al. (2018). “XPS study of cobalt-ceria catalysts for ammonia synthesis–The reduction process.” Vacuum 155: 434-438. Read it online here.
- Van Campen, D. and L. Klebanoff (1994). “Spin-resolved and high-energy-resolution XPS studies of the 3s and 2s levels of metallic cobalt.” Physical Review B 49(3): 2040. Read it online here.
- Carver, J., et al. (1972). “Use of X‐Ray photoelectron spectroscopy to study bonding in Cr, Mn, Fe, and Co compounds.” The Journal of chemical physics 57(2): 973-982. Read it online here.
- Brundle, C., et al. (1976). “X-ray photoemission study of the interaction of oxygen and air with clean cobalt surfaces.” Surface science 60(2): 286-300. Read it online here.