
Tantalum
Doublet Separations
- Ta 4f: 1.92 eV
- Ta 4d: 11.5 eV
- Ta 4p: 62.5 eV
The Energies Listed are Binding Energies!
- Ta 4s: 566 eV
- Ta 4p: 405 eV
- Ta 5d: 230 eV
- Ta 5s: 71 eV
- Ta 5p: 37 eV
- Ta 4f: 22 eV
- Ta 5d: 6 eV
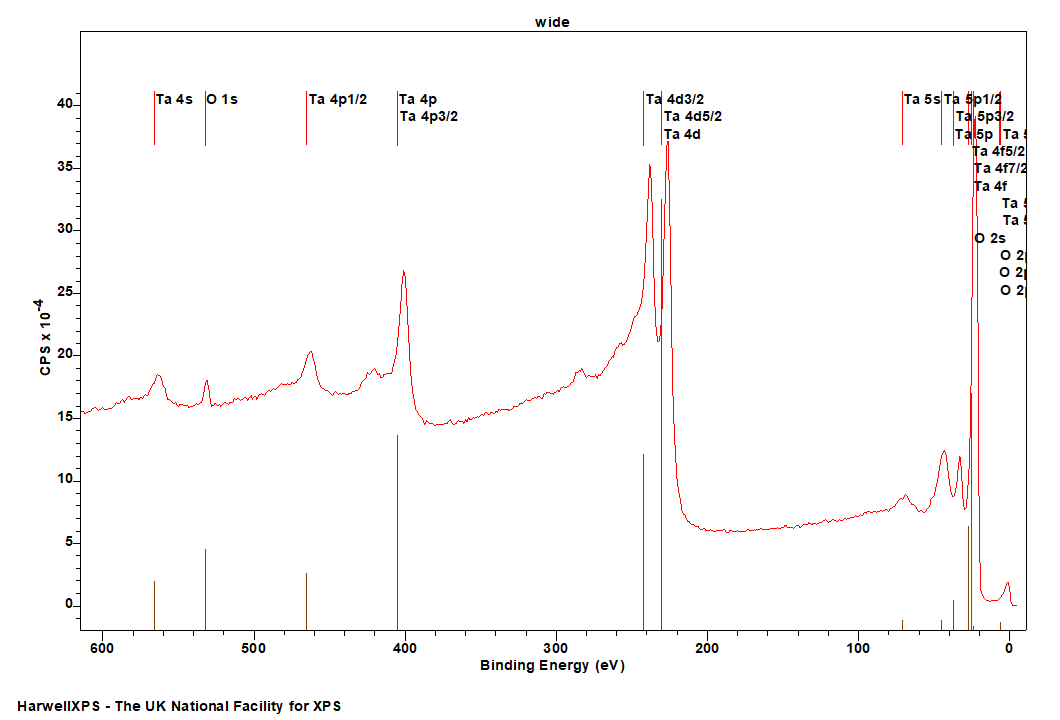
The Energies Listed are Binding Energies!
Ta is primarily analysed via the 4f orbital
Due to the low energy of Ta 4f, there are many potential peak overlaps, so we will only list the most problematic.
- O 2s – should be possible to deconvolute, but significant overlap
- F 2s – mostly problematic for oxidised Ta
- Na 2p – mostly problematic for oxidised Ta
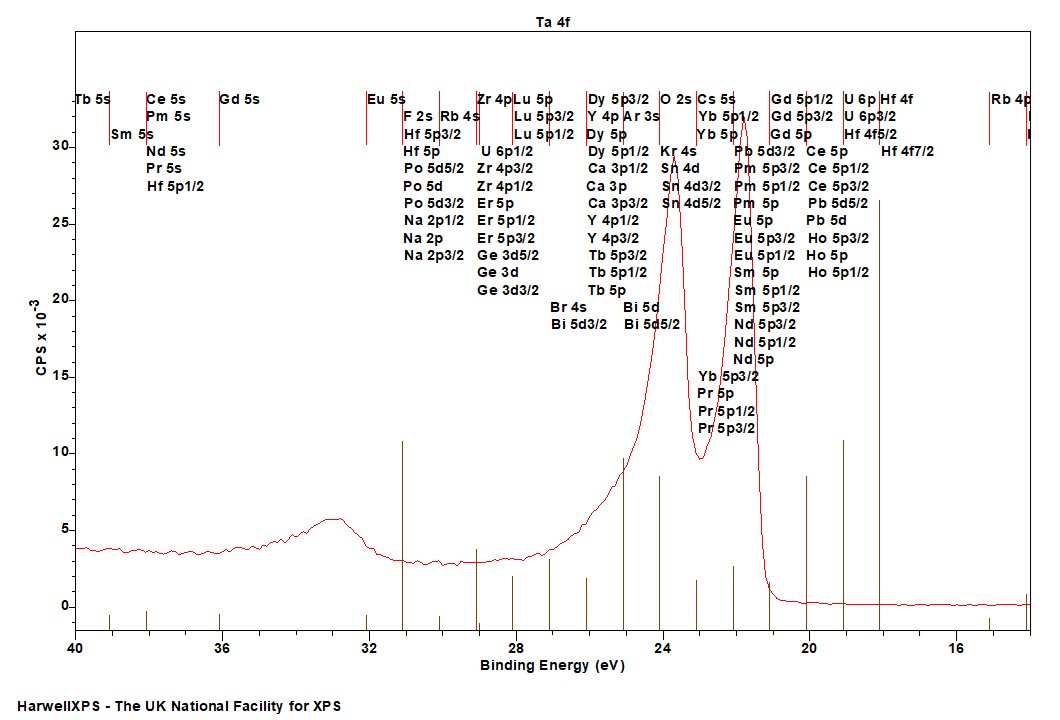
Energies listed are Kinetic Energies!
Ta NNN (Coster-Kronig): ~ 168 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| Ta(0) | 21.5 | Au 4f (83.98 eV) | 1 |
| Ta2O5 | 27.0 ± 0.05 | Au 4f (83.98 eV) | 1 |
| Ta (I) suboxide | 22.5 ± 0.10 | Au 4f (83.98 eV) | 1 |
| Ta (II) suboxide | 23.3 ± 0.30 | Au 4f (83.98 eV) | 1 |
| Ta (III) suboxide | 24.3 ± 0.20 | Au 4f (83.98 eV) | 1 |
| Ta (IV) suboxide | 25.7 ± 0.30 | Au 4f (83.98 eV) | 1 |
Tantalum (Ta) is highly valued for its exceptional corrosion resistance, high melting point, and biocompatibility, making it an essential material in various industries. Its remarkable resistance to oxidation and chemical attack, even in highly aggressive environments, makes it ideal for chemical processing equipment, heat exchangers, and aerospace components. Tantalum’s high conductivity and stability also make it crucial in the production of electronic capacitors used in mobile phones, computers, and automotive electronics. Due to its biocompatibility, tantalum is widely used in medical implants, such as orthopedic and dental applications, where it promotes bone integration. Additionally, its ability to form a stable oxide layer (Ta₂O₅) enhances its performance in thin-film coatings, superconductors, and optical applications.
Tantalum XPS analysis is traditionally performed on the 4f region due to the high intensity and readily resolved peaks. Ta 4f presents a typical f-orbital doublet shape with tantalum oxide and sub-oxides appearing at well separated intervals.
Tantalum 4f lies very close to the Ta 5s feature, and both should be collected in order to properly assess the background (up to ~ 40 eV).
Tantalum oxide degrades quickly under ion beam etching to form many suboxides.[1]
Tantalum metal exhibits asymmetric peaks shapes, and lies over the O 2s feature, making analysis of large amounts of oxide pottentially tricky. 
Not available
Not available
- Simpson, Robin, et al. “XPS investigation of monatomic and cluster argon ion sputtering of tantalum pentoxide.” Applied Surface Science 405 (2017): 79-87. Read it online here.