XPS for Beginners
- Fundamentals of XPS
- Data Analysis
- Applications of XPS
- Related Techniques
Upon exiting the atom, a photoelectron may promote the resulting ion into a excited state
This process costs the photoelectron an amount of kinetic energy, meaning it will be recorded as if it possessed a higher binding energy within the atom
The peaks produced by this method are called ‘Shake-up satellites’

For transition metals, such as Cu, the shake-up can be described as a strong interaction of the final states
This involves charge transfer from the O 2p band to the Cu 3d band
This effectively results in a final configuration of 2p53d9
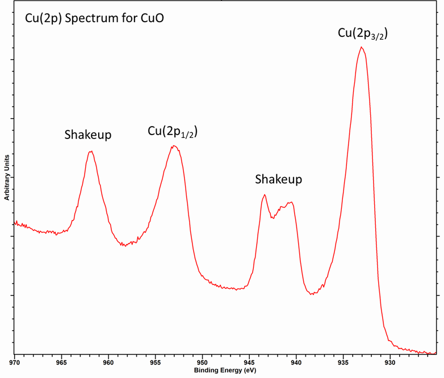

Metals and conductive materials may exhibit a special type of shake-up process caused by the conduction band
The continuum of energies produces photoelectrons which lose a corresponding continuum of kinetic energy
Results in peaks which exhibit extended tails on the high binding energy side

Degree of asymmetry depends heavily on DOS near Fermi level
High DOS = more of a continuum for shake-ups
E.g. Au and Pt

Another good example is first row TMs
As we fill the d-band we lose asymmetry

Materials with high density of free electrons around the Fermi level may form plasmon peaks
Plasmons are oscillations in the ‘sea’ of electrons around the Fermi level
Photoelectrons may interact with this plasmon upon exiting the material and lose a defined amount of energy

Interval between plasmons constant
Sometimes plasmonic peaks arising from surface plasmons and bulk plasmons may be separated
Al, Mg, Si, Na typically exhibit strong plasmon structure
Following photoemission, the atom will respond to the newly created core hole
This response results in so-called ‘final state effects’
E.g. Lanthanum – produces 2 sets of doublets by XPS

2 peaks near 835 eV = 3d5/2
2 peaks near 850 = 3d3/2
Higher binding energy peaks = unshielded core emission
Lower binding energy peaks = shielded core emission
Following photoemission, Lanthanum may rearrange to reduce the total energy of the system via charge transfer from the conduction band to the 4f orbitals forming the configuration: 4fn+1(3dn+1) – cf1L
Alternatively, the system may stay in the ground state: 4fn(3dn) – cf0 – resulting in a higher binding energy peak
Core hole notation: c = La core hole, L = ligand core hole

Unpaired electrons may cause multiplet structure in XPS spectra
Coupling with the newly created core-hole creates a number of final-states resulting in multiple emission peaks
First-row transition metals especially affected

This complicates data analysis greatly
E.g. Ni free ion envelopes (i.e. no final-state effects from ligands)
Many peaks arise due to unpaired electron coupling

These multiplet fits combined with shake-up satellites and other final state effects form the complex structures seen in first row TM compounds
It is often advised to analyse these spectra qualitatively where possible
Procedures for chemical deconvolution exist – but are hard!