
Lead
Doublet Separations
- Pb 4f: 4.9 eV
- Pd 4d: 22.1 eV
- Pb 4p: ~118 eV
- Pb 5d: 2.6 eV
- Pb 5p: 22.4 eV
The Energies Listed are Binding Energies!
- Pb 4s: 894 eV
- Pb 4p: 645 eV
- Pb 4d: 413 eV
- Pb 4f: 138.6 eV
The Energies Listed are Binding Energies!
Pb is primarily analysed via the 4f orbital
- Sm 4d (130 eV)
- P 2p (130 eV)
- Se LMM b (Al source) (134 eV)
- Eu 4d (134 eV)
- Sr 3d (136 eV)
- Sn 4s (137 eV)
- Zn 3s (137 eV)
- As 3p (141 eV)
- Gd 4d (141 eV)
- Xe 4d (147 eV)
- Tb 4d (148 eV)
- Sb 4s (152 eV)
- Si 2s (153 eV)
Energies listed are Kinetic Energies!
Pb NOO: ~ 92 eV
The Energies Listed are Binding Energies!
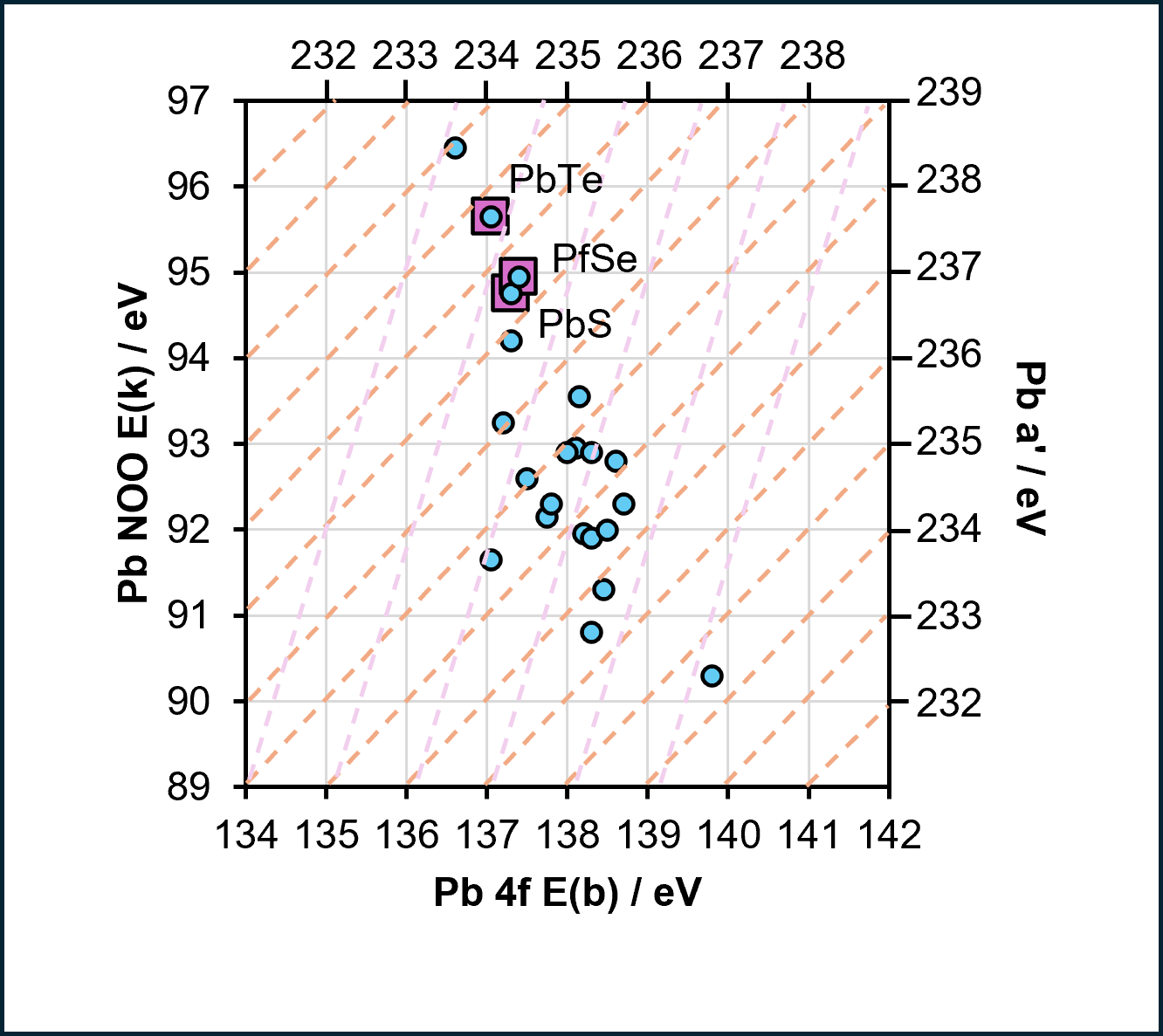
| Species | Binding energy / eV | Charge Ref | Ref |
| Pb(0) | 136.6 | Au 4f (83.8 eV) | 1 |
| PbF2 | 138.3 | C 1s (284.6 eV) | 1 |
| PbCl2 | 138.7 | C 1s (284.6 eV) | 1 |
| PbBr2 | 138.6 | C 1s (284.6 eV | 1 |
| PbI2 | 138.15 | C 1s (284.6 eV | 1 |
| PbO | 137.5 | C 1s (284.6 eV | 1 |
| PbS | 137.3 | C 1s (284.6 eV | 1 |
| PbSe | 137.4 | C 1s (284.6 eV | 1 |
| PbTe | 137.05 | C 1s (284.6 eV | 1 |
| PbAc2 | 137.05 | C 1s (284.6 eV | 1 |
| 2PbCO3.Pb(OH)2 | 138.2 | C 1s (284.6 eV | 1 |
| PbCrO4 | 138.1 | C 1s (284.6 eV | 1 |
| Pb(IO4)2 | 138 | C 1s (284.6 eV | 1 |
| PbNCN | 137.3 | C 1s (284.6 eV | 1 |
| Pb(NO3)2 | 138.3 | C 1s (284.6 eV | 1 |
| PbO2 | 137.2 | C 1s (284.6 eV | 1 |
| Pb(OH)2 | 137.75 | C 1s (284.6 eV | 1 |
| PbSiO3 | 138.45 | C 1s (284.6 eV | 1 |
| PbSO4 | 139.8 | C 1s (284.6 eV | 1 |
| PbTiO3 | 137.8 | C 1s (284.6 eV | 1 |
| PbWO4 | 138.5 | C 1s (284.6 eV | 1 |
| PbZrO3 | 138.3 | C 1s (284.6 eV | 1 |
Lead compounds may be difficult to distinguish, particularly those of the oxides, due to similarities in binding energy. It is often advisable to record the Pb MNN auger to assist in speciation. (You can download the raw data for these Wagner plots in the supplementary section).




Pb(0) artefacts may be caused by X-ray photolysis, which induces the reduction of Pb(II) to Pb(0) under typical analytical conditions. Additionally, chemical changes due to film aging in air and the presence of PbI2 in the perovskite films can facilitate in situ reactions that generate these artefacts. To prevent Pb(0) artefacts, it is important to minimize X-ray exposure time to limit photolysis effects, perform XPS analysis on freshly prepared samples to reduce the impact of film aging, and conduct the analysis under a clean ultra-high vacuum (UHV) conditions to mitigate the influence of environmental factors.
See our article on X-ray damage for more information on preventing this.
Pb 4f peaks display symmetric, gaussian-like peaks, and do not display any particular irregularities.
Not available
- Pederson, L. R. “Two-dimensional chemical-state plot for lead using XPS.” Journal of Electron Spectroscopy and Related Phenomena 28.2 (1982): 203-209. Read it online here.