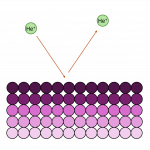
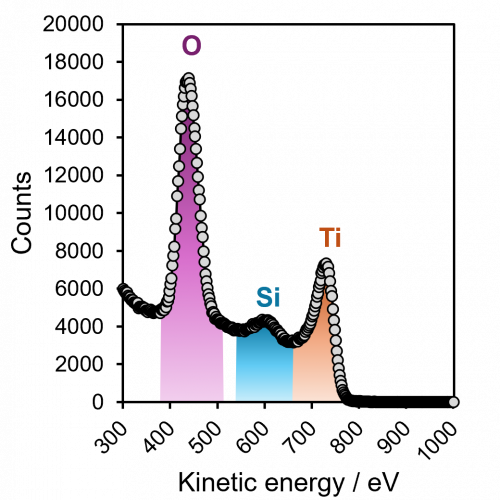
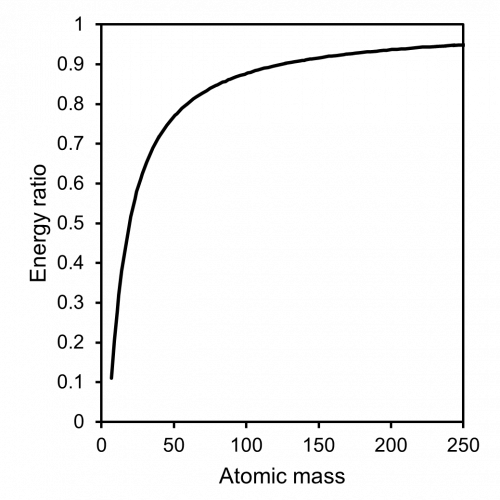
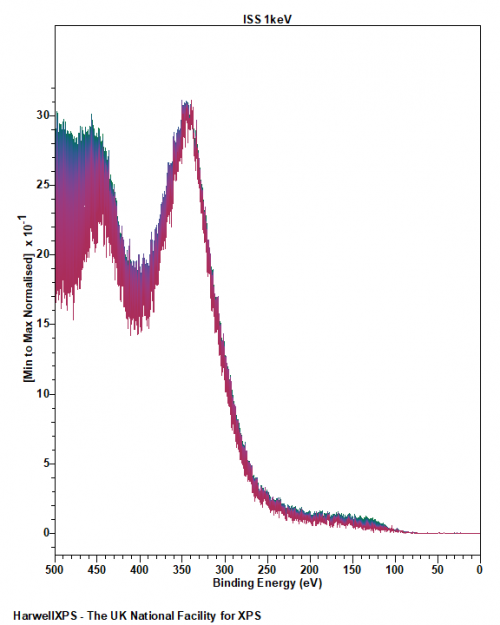
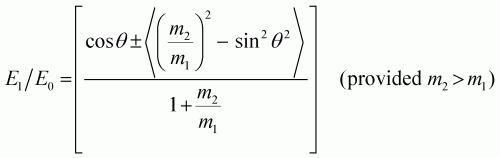Ion scattering spectroscopy (ISS), also known as low energy ion scattering (LEIS), is a surface technique which involves probing the outermost surface layers of a solid using elastic collisions of ions. While ISS is generally be used to provide surface identities and ratios, it may also be used in a semi-quantitative manner through comparison with standard samples. Originally involving high energy (MeV) ions (and often referred to as Rutherford Backscattering Spectroscopy or RBS), the ISS technique was refined to utilise lower energy ions to be more readily available in laboratories. It was the discovery that ion-surface collisions could be described kinematically by a simple two body elastic collision model that allowed for facile determination of surface composition:
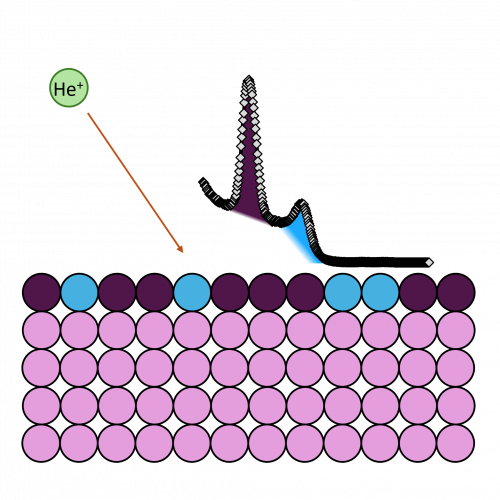
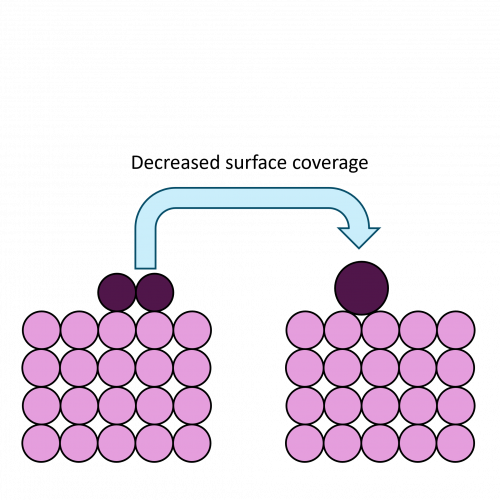
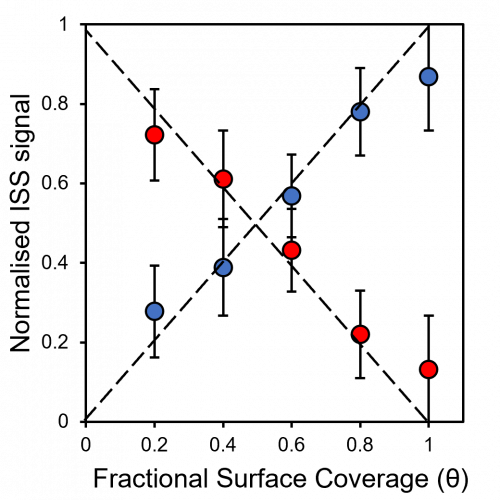
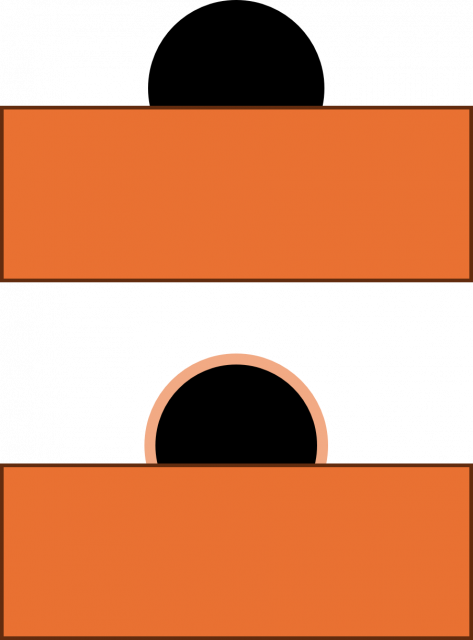
The extreme surface sensitivity of ISS allows it to offer additional structural information, complementary to that of pure XPS analysis and it has found use in many fields. While ISS remains a valuable tool in materials analysis, care needs to be taken over sample choice and preparation. For example, ‘rough’ surfaces such as silicas commonly used in heterogeneous catalysis may exhibit broadened peaks in eventual ISS profiles. To overcome this, compacting samples into pellets may improve surface suitability, however, care must be taken so as not to alter the material properties. Furthermore, ion scattering may have limitations depending on sample composition, since preferential sputtering can lead to erroneously low concentrations of easily sputtered components such as sodium.