Auger-Meitner peaks often offer additional insight into the chemical environment of an atom or element, particularly in cases where distinction of chemistry is tricky to determine by core-electron peaks alone (e.g. Copper, Silver, Zinc). Analysis of the auger peaks may also offer more detailed information into the electronic configurations for those interested in more than just the system chemistry.
The Auger, or Auger-Meitner, Process and Auger Peaks
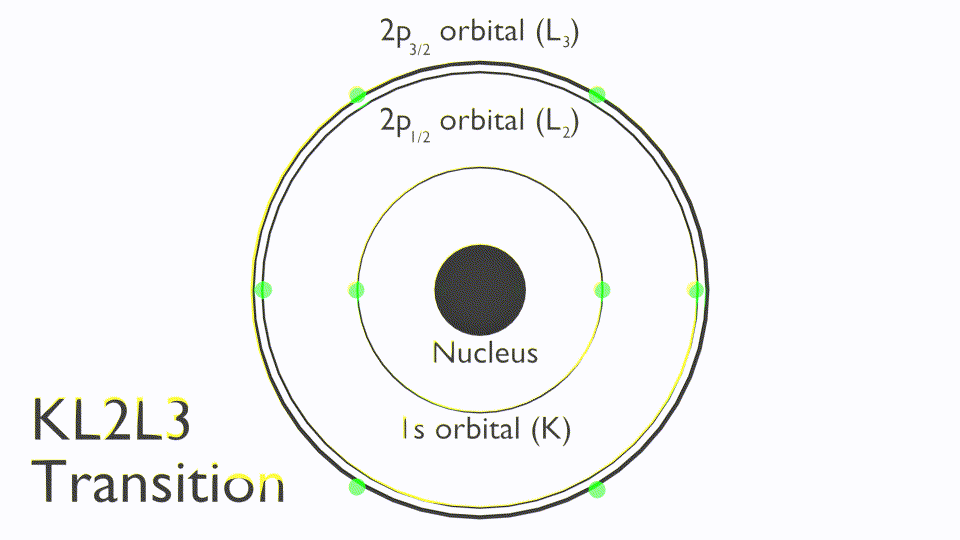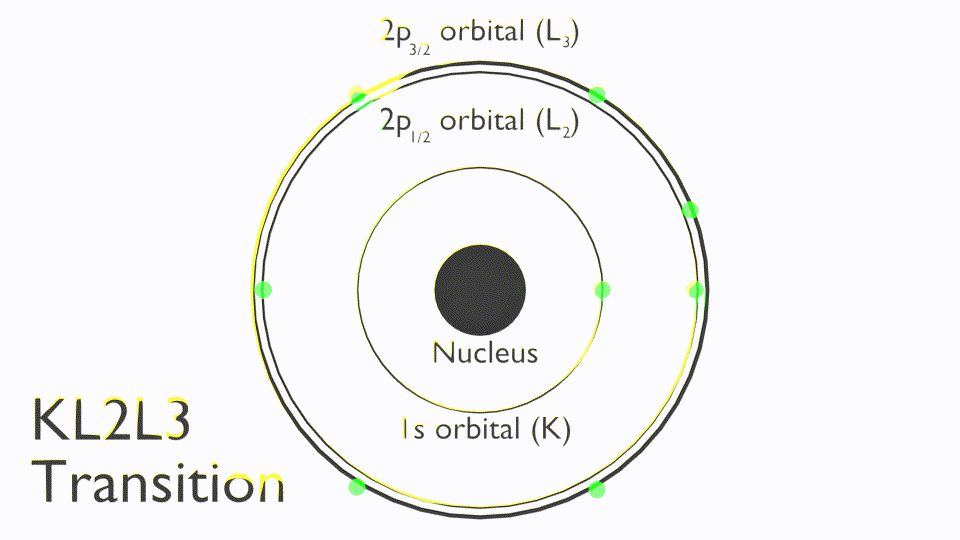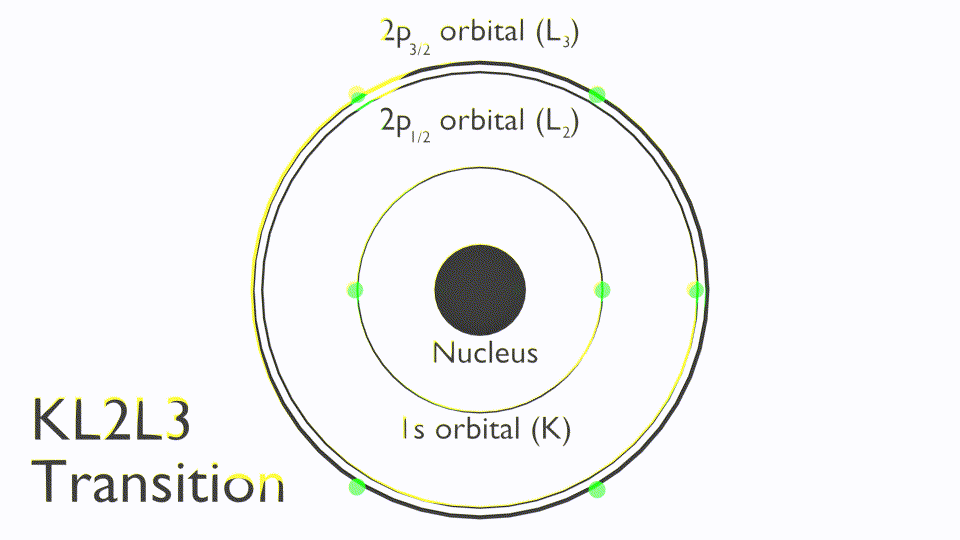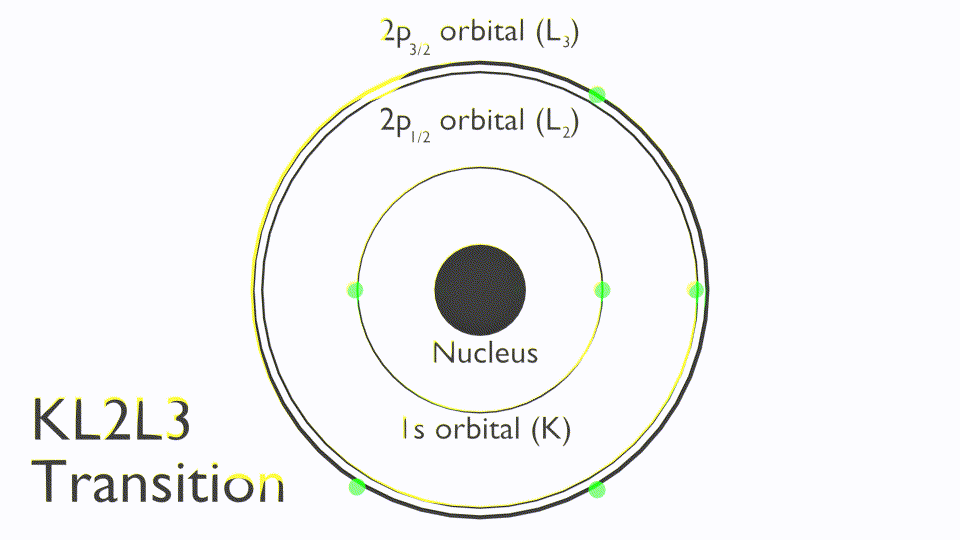
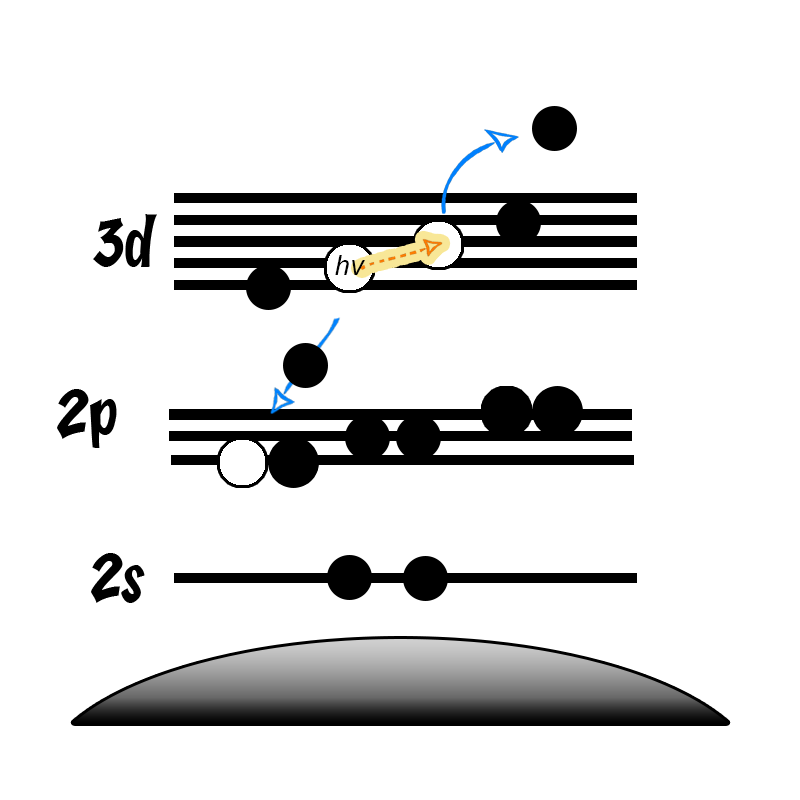
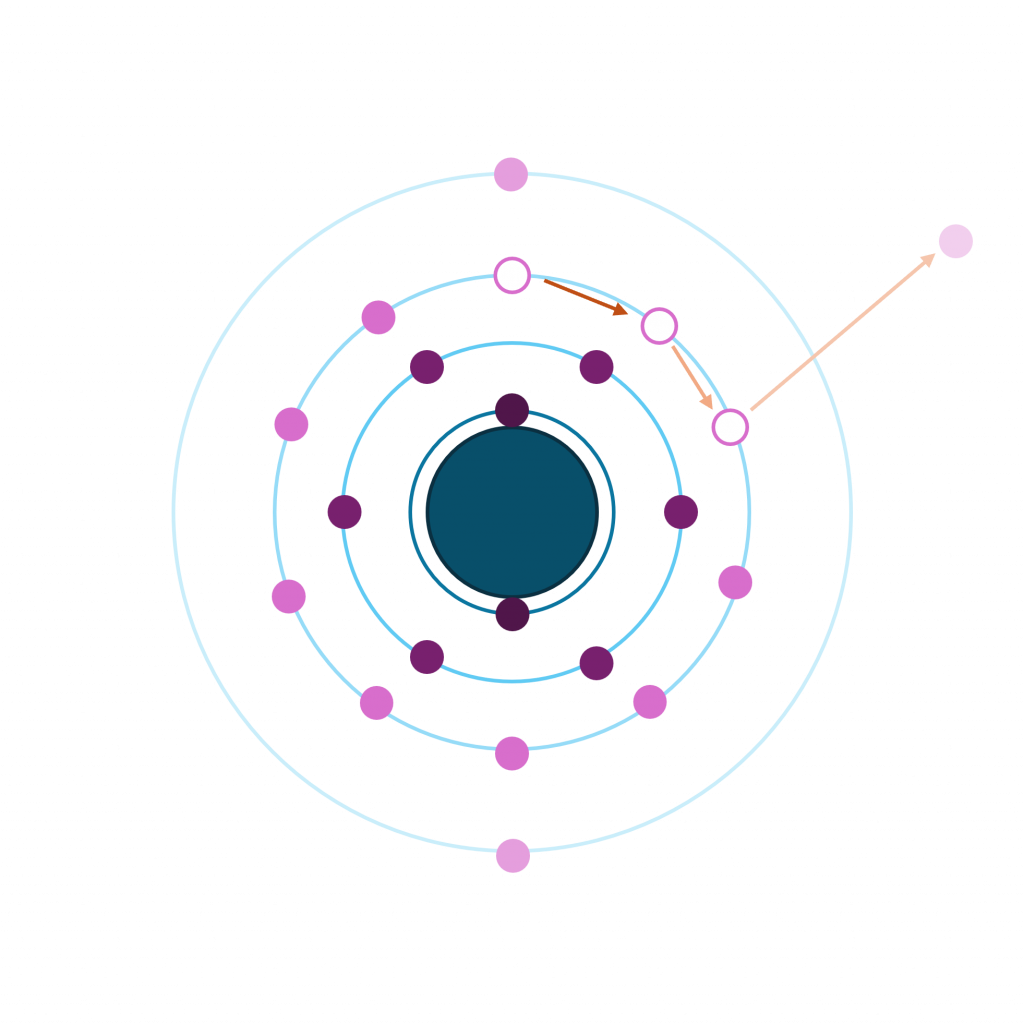
The Auger process involves the filling of an inner-shell vacancy which results in the emission of an electron from within the same atom. The Auger process takes place in three steps:
- Photoemission of a core electron to leave a core hole (ionization)
- A radiationless transition where an electron from a higher orbital falls to fill the core-hole (relaxation)
- The excess energy of the exited state ion is removed by the ejection of an Auger electron (emission)
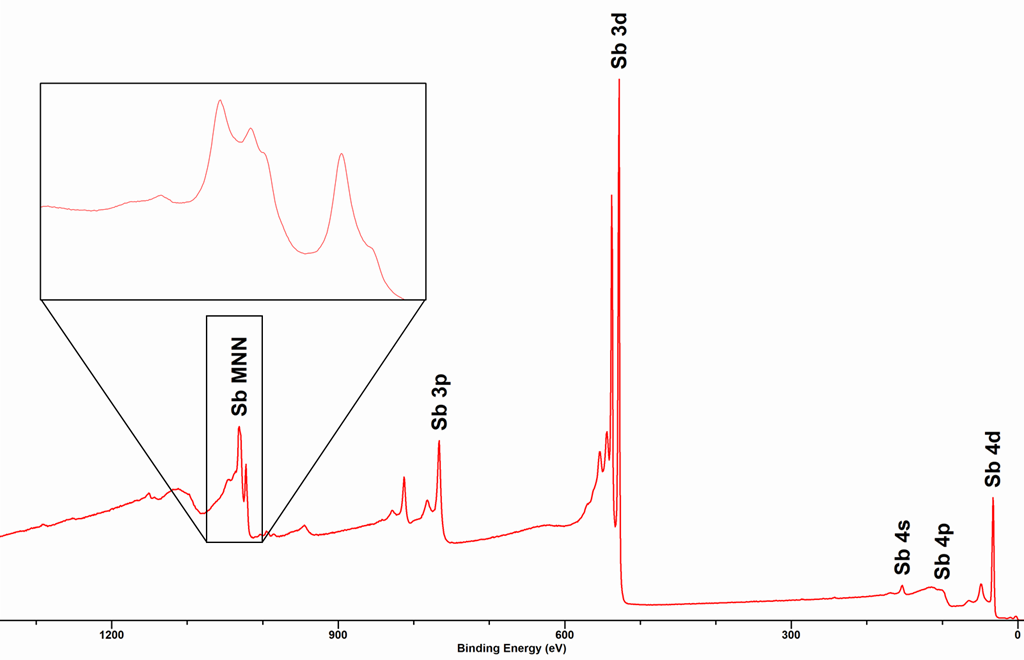
 Due to this three elcetron process, the shapes of Auger peaks are typiclly more complex than those of most core-levels. An example of an Auger peak is shown below for the MNN Auger peaks (see insert) for metallic Sb, together with the core-level peaks.
Due to this three elcetron process, the shapes of Auger peaks are typiclly more complex than those of most core-levels. An example of an Auger peak is shown below for the MNN Auger peaks (see insert) for metallic Sb, together with the core-level peaks.
Note that Auger transitions are typically reported a kinetic energy not binding energy and use X-ray notation (K,L,M,N) to state the orbitals involved in the Auger process, these are termed as follows:
| Auger Notation | XPS Notation |
| K | 1s1/2 |
| L1 | 2s1/2 |
| L2 | 2p1/2 |
| L3 | 2p3/2 |
| M1 | 3s1/2 |
| M2 | 3p1/2 |
| M3 | 3p3/2 |
| M4 | 3d3/2 |
| M5 | 3d5/2 |
Notation for Augers involving 4s orbitals and above are detailed in the “Handbook of Applied Solid State Spectroscopy” by Springer
CasaXPS