
Bismuth
Doublet Separations
- Bi 4f: 5.3 eV
- Bi 4d: 23.5 eV
- Bi 4p: 126.6 eV
- Bi 5d: 3.1 eV
- Bi 5d: 26.3 eV
The Energies Listed are Binding Energies!
- Bi 4s: 939 eV
- Bi 4p: 679 eV
- Bi 4d: 440 eV
- Bi 4f: 158 eV
- Bi 5s: 160 eV
- Bi 5p: 93 eV
- Bi 5d: 25 eV
- Bi 6s: 8 eV
- Bi 6p: 3 eV
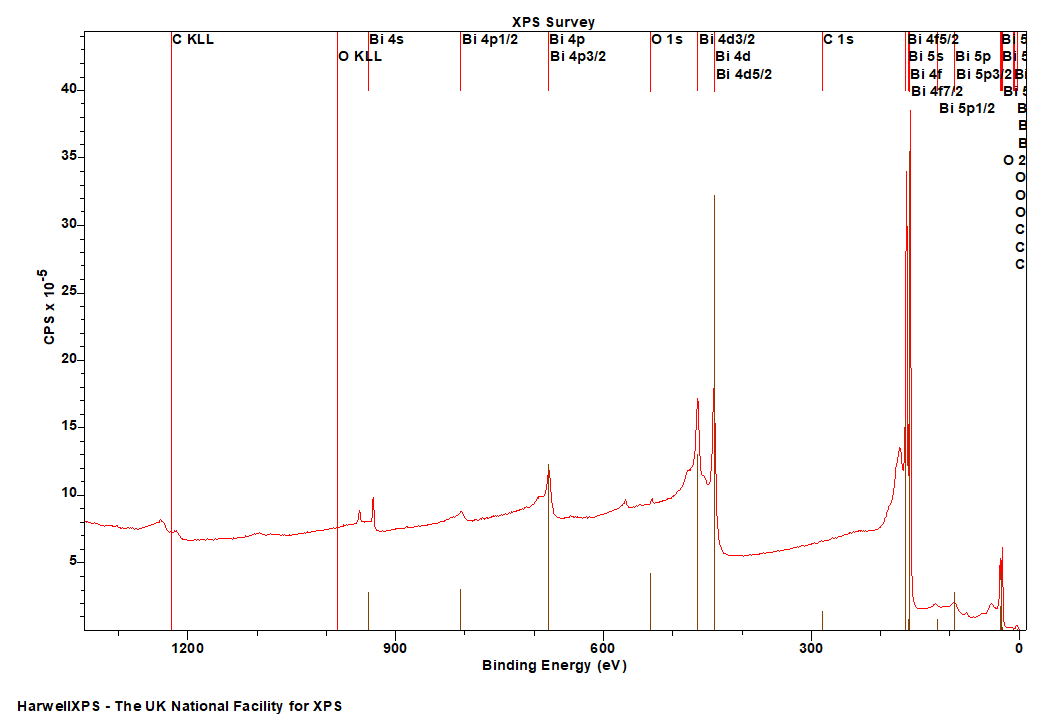
The Energies Listed are Binding Energies!
Bi is primarily analysed via the 4f orbital
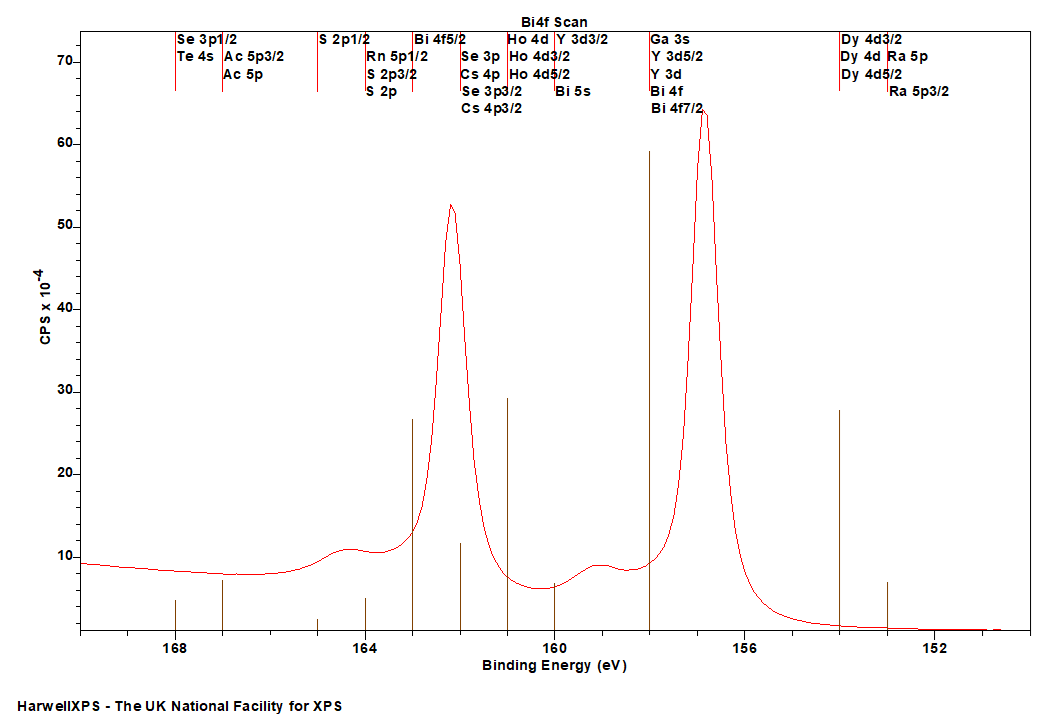
- Ra 5p: 153 eV
- Dy 4d: 154 eV
- Ga 3s: 158 eV
- Y 3d: 158 eV
- Ho 4d: 161 eV
- Se 3p: 162 eV
- Cs 4p: 162 eV
- S 2p: 164 eV
- Ac 5p: 167 eV
- Te 4s: 168 eV
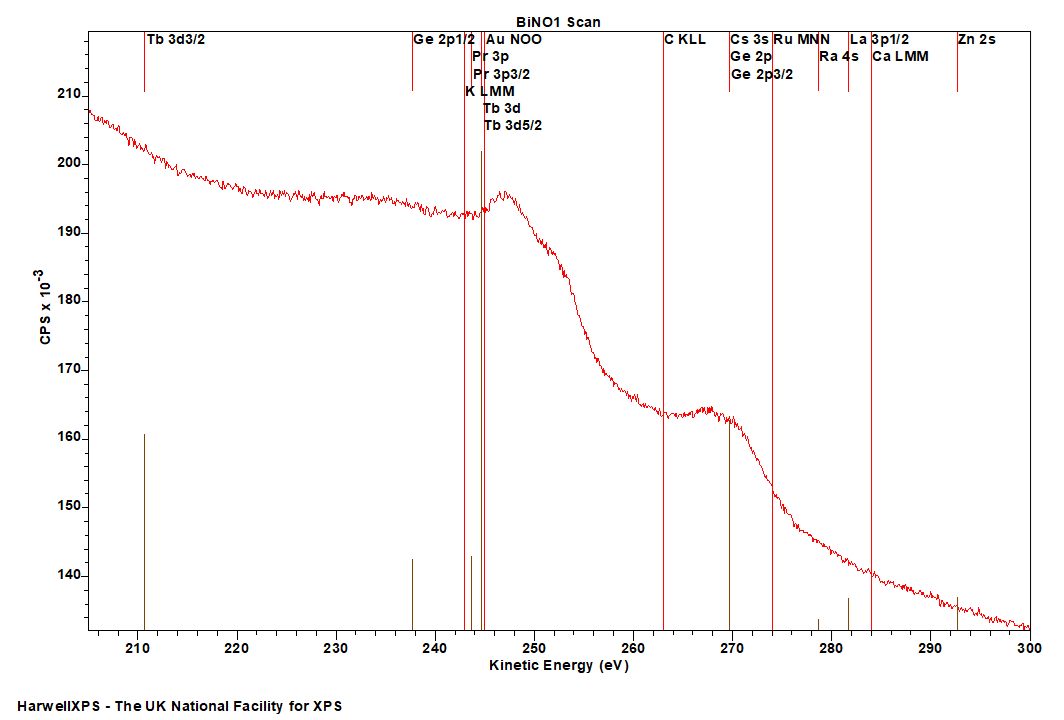
Energies listed are Kinetic Energies!
Bi NOO: ~ 100-200 eV
Bismuth (Bi) is a brittle, lustrous metal with a gray-white colour and a slight reddish tinge. It is the most metallic and least abundant element in the nitrogen group. Bismuth is used in a variety of applications due to its unique properties. It is found in cosmetics, pharmaceuticals, and as a non-toxic replacement for lead in products like plumbing and ammunition. Bismuth alloys, such as Wood’s metal, are used in fire detection and suppression systems because they melt at low temperatures. Surface analysis of bismuth is crucial because it helps understand the material’s surface chemistry, which influences its reactivity, adhesion, and corrosion resistance. Techniques like XPS provide detailed information about the elemental composition and chemical states of the surface, which is essential for optimizing its use in various applications.
Bismuth (III) is known to reduce when exposed to X-rays for a significant period,[4] so it would be advisable to perform reduction checks (single scans before and after the experiment) to check the bismuth is not affected by the measurement.
See our page on sample damage for more information.
Ion etching may also reduce bismuth species during depth profiles to the metal following prolonged exposure.[5] Keep this in mind when performing depth profiles, and potentially consider options to account for this reduction (e.g. HAXPES).
If sulfur is present, it may be helpful to record the S 2s peak too to help account for the S 2p – Bi 4f overlap.
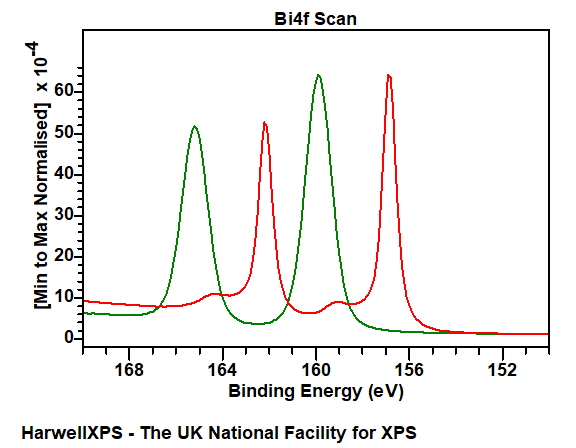
Bi metal does not exhibit a huge amount asymmetry, while oxides and compounds are symmetric in nature.

Not available
Not available
- Dharmadhikari, Vineet S., et al. “Characterisation of thin films of bismuth oxide by X-ray photoelectron spectroscopy.” Journal of Electron Spectroscopy and Related Phenomena 25.2 (1982): 181-189. Read it online here.
- Rodriguez-Pereira, Jhonatan, Sergio A. Rincón-Ortiz, and Rogelio Ospina. “Bismuth acetate by XPS.” Surface Science Spectra 27.2 (2020). Read it online here.
- Shallenberger, Jeffrey R., et al. “2D bismuth telluride analyzed by XPS.” Surface Science Spectra 26.2 (2019). Read it online here.
- Süzer, Şefik. “XPS investigation of X-ray-induced reduction of metal ions.” Applied Spectroscopy 54.11 (2000): 1716-1718. Read it online here.
- Zhi-Cheng, Jiang, An Li-Dun, and Yin Yuan-Gen. “XPS study of ion-induced chemical effect on β-bismuth molybdate catalyst.” Applied surface science 24.2 (1985): 134-146. Read it online here.