
Bromine
Doublet Separations
- Br 3d: 1.04 eV
The Energies Listed are Binding Energies!
- Br 3s: 254 eV
- Br 3p: 182 eV
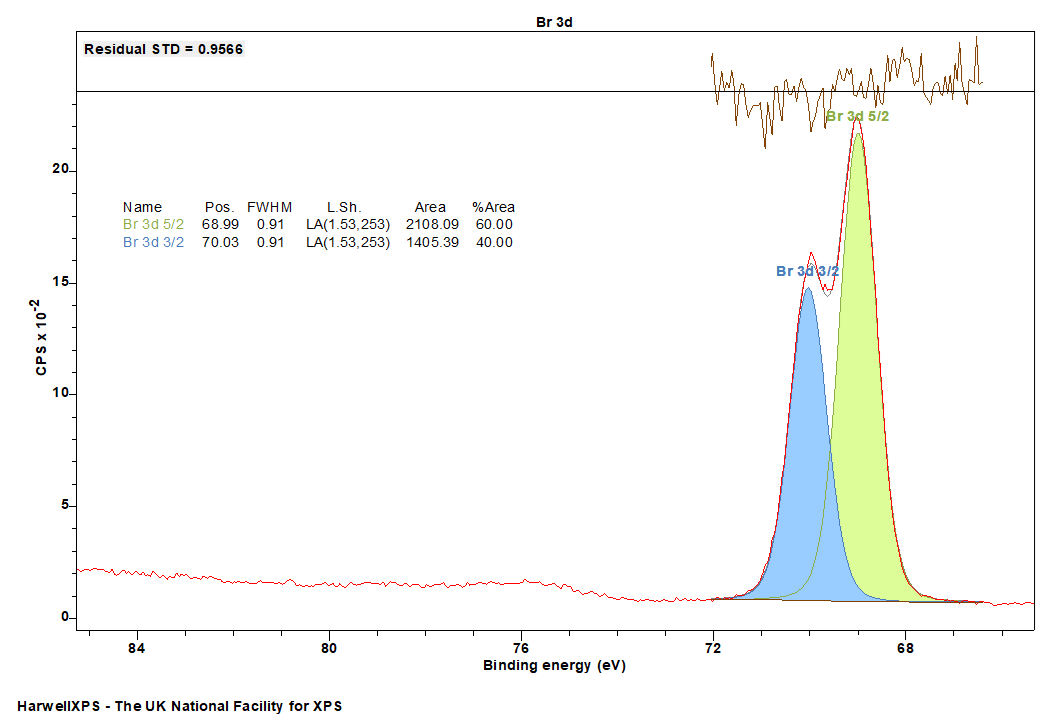
- Br 3d: 69 eV
- Br 4s: 27 eV
- Br 4p: 5 eV

The Energies Listed are Binding Energies!
Br is primarily analyzed via the 3d orbital
- Cd 4p: 67 eV
- Ra 5d: 68 eV
- Ni 3p: 68 eV
- Pt 4f: 70 eV
- Ta 5s: 71 eV
- Au 5p: 72 eV
- Al 2p: 73 eV
- Cr 3s: 74 eV
- Cu 3p: 74 eV
- Ru 4s: 75 eV

Energies listed are Kinetic Energies!
Br LMM: ~ 1390 eV
Bromine (Br) is a deep red, volatile liquid at room temperature and is part of the halogen group in the periodic table. It is highly reactive and has a variety of applications. Bromine is widely used as a flame retardant in textiles, plastics, and electronic devices, helping to reduce flammability. It is also employed as a disinfectant for swimming pools and spas, where it effectively kills bacteria, viruses, and other pathogens. Additionally, bromine compounds are used in the production of pharmaceuticals, agricultural chemicals, and photographic films. Surface analysis of bromine is important because it helps understand its reactivity and interactions with other materials. Techniques like XPS provide detailed information about the elemental composition and chemical states of bromine on surfaces, which is crucial for optimizing its use in various applications.
X-ray exposure has been found to degrade brominated organics, and so it is advised to record a pre- and post- analysis scan of the Br 3d region to check for sample damage of a new material.[2]
See our page on sample damage for more advice on dealing with sample damage.
Br 3d can be readily fir with a Shirley background and Voigt-type LA lineshapes.
Not available
Not available
- Morgan, Wayne E., John R. Van Wazer, and Wojciech J. Stec. “Inner-orbital photoelectron spectroscopy of the alkali metal halides, perchlorates, phosphates, and pyrophosphates.” Journal of the American Chemical Society 95.3 (1973): 751-755. Read it online here.
- Al‐Bataineh, Sameer A., Leanne G. Britcher, and Hans J. Griesser. “Rapid radiation degradation in the XPS analysis of antibacterial coatings of brominated furanones.” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 38.11 (2006): 1512-1518. Read it online here.