
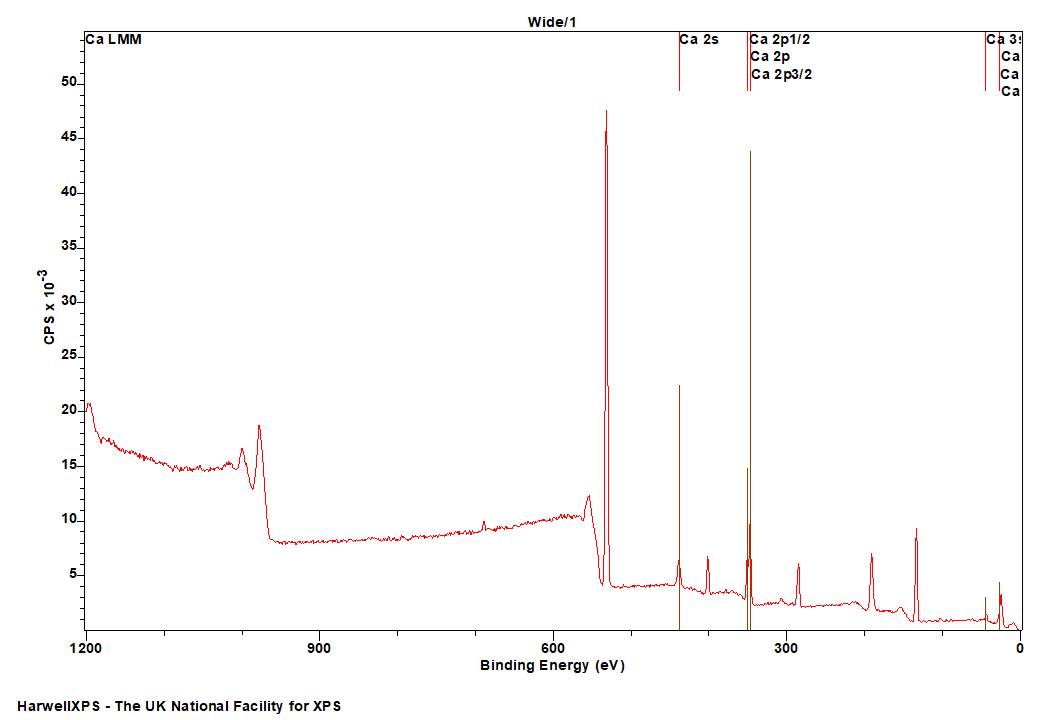
Calcium
Doublet Separations
- Ca 2p: 3.5 eV
- Ca 3p: 0.4 eV
The Energies Listed are Binding Energies!
- Ca 2s: 438 eV
- Ca 2p: 347 eV
- Ca 3s: 44 eV
- Ca 3p: 26 eV
The Energies Listed are Binding Energies!
Ca is primarily analysed via the 2p orbital
- Pd 3d (340 eV)
- Ge LMM (Al source) (341 eV)
- Ho 4p (343 eV)
- Yb 4p (343 eV)
- Th 4f (344 eV)
- Zr 3p (345 eV)
- Sm 4s (347 eV)
- Au 4d (352 eV)
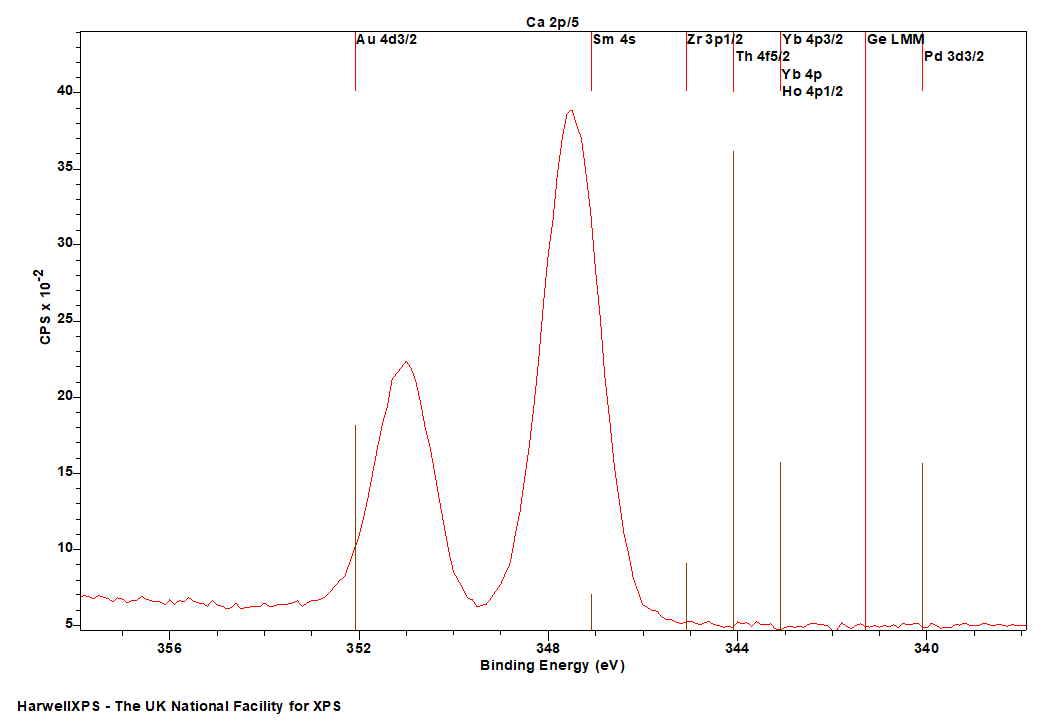
Energies listed are Kinetic Energies!
Ca LMM: ~ 284 eV
The Energies Listed are Binding Energies!
Calcium is crucial in materials science due to its role in structural materials, biomaterials, ceramics, and electronics. It is a key component in cement (CaO, CaCO₃), bioceramics like hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂), and functional materials such as CaF₂ for optics and CaTiO₃ for dielectrics.
Calcium compounds show subtle differences in binding energies and can therefore be slightly difficult to distinguish.
Calcium is often seen as a contaminant on poorly handled samples. If calcium is found unexpectedly, it is possible the sample surface was touched without gloves on.
If recording a sample containing Mg, record the Ca 2s for quantification as Mg KLL overlaps with Ca 2p.
Calcium rarely exists in it’s metallic form, and as such typically peak shapes are found to be symmetric.
Not available
Not available
- Powell, C. J. “Elemental binding energies for X-ray photoelectron spectroscopy.” Applied Surface Science 89.2 (1995): 141-149. Read it online here.
- Hanawa, Takao, and Mamoru Ota. “Calcium phosphate naturally formed on titanium in electrolyte solution.” Biomaterials 12.8 (1991): 767-774. Read it online here.
- Engelhard, Mark, and Don Baer. “Vacuum cleaved calcium carbonate by XPS.” Surface Science Spectra 6.2 (1999): 153-159. Read it online here.
- Briggs, David. “Handbook of X-ray and ultraviolet photoelectron spectroscopy.” (No Title) (1977). Read it online here.