Cerium
Doublet Separations
- Ce 3d: 18.6 eV
- Ce 4d: 3.2 eV
The Energies Listed are Binding Energies!
Ce 3p: 1190 eV
Ce 3d: 888 eV
Ce 4s: 293 eV
Ce 4p: 215 eV
Ce 4d: 112 eV
The Energies Listed are Binding Energies!
Overlaps for Ce 3d (primary emission)
- Sn 3s: 884 eV
- At 4p: 886 eV
- Ac 4p: 890 eV
- Pb 4s: 894 eV
- Ba MNN (Al kα X-rays) (903 eV)
- Mn LMM (Al kα X-rays) (905 eV)
Overlaps for Ce 4d
- Cd 4s (108 eV)
- Au 5s (108 eV)
- Te 4p (110 eV)
- Be 1s (111 eV)
- Rb 3d (111 eV)
- Ni 3s (112 eV)
- Pr 4d (114 eV)
- At 5p (115 eV)
- Bi 5p (117 eV)
- Nd 4p (118 eV)
- Al 2s (118 eV)
Energies listed are Kinetic Energies!
Ce MNN: ~ 658 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| Ce2O3 | 880 eV | C 1s (284.5 eV) | 8 |
| CeO2 | 882 eV | C 1s (284.5 eV) | 8 |
Assigning cerium states based on binding energy alone is not recommended due to the complex final state effects involved
Cerium is a very useful material in heterogeneous catalysis and other fields due to it’s ability to store and move oxygen, but the XPS spectrum is not straightforward to deconvolute due to complications arising from final-state effects. Typically, cerium is observed as either Ce(III) or Ce(IV) and both states produce complex spectra. There is a significant spin-orbit separation between the 3d5/2 and 3d3/2 emission features (Δ = 18.6 eV)

Figure 1: Deconvolution of a mixed cerium oxide indicating Ce 3+ (green) and Ce 4+ (blue) envelopes
Trivalent Ce (e.g. Ce2O3):
Ce3+ has a ground state of 4f1 5d0 6s0 but following photoemission of a 3d core electron may split into two final states. One is the poorly screened 4f1 ground state (higher energy) and the other is a well screened 4f2 state, following a charge transfer process. Including spin-orbit coupling this leaves us with 4 peaks for the 3+ state.1
Quadravalent Ce (e.g. CeO2)
Ce4+ was typically thought to have a ground state of 4f0, however XPS analysis of CeO2 recorded 3 peaks (6 peaks taking into consideration the spin-orbit coupling effect), similar to a range of mixed valence compounds such as αCe, CePd3 and CeRh3. From this, it was determined that quadrivalent Ce is actually a mixture of ground states – 4f0, 4f1L and 4f2L2 (L is a hole in the O 2p band) due to strong covalency hybridisation between Ce 4f and O 2p.1
Cerium presents a difficulty in it’s analysis, due to the propensity of Ce 4+ to reduce to Ce 3+ when under the exposure of X-rays in a UHV system. Ce 4+ may also reduce when exposed to vacuum alone (albeit more slowly).8

Cerium oxide, exposed to X-rays for 60 minutes.
Charge neutralisation systems have not been shown to impact cerium oxidation state, however the gun setting used to generate the X-rays involved do affect the rate of reduction of cerium. When measuring Ce, it is useful to minimise the X-ray power as much as practically possible.
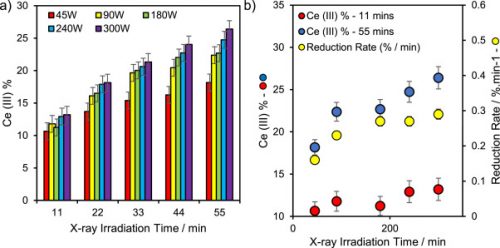
Ce reduction as a function of X-ray power.
Modern instruments offer automated height optimisation functions – however caution must be used for this since it involves turning on the X-rays. It is advisable to do this on a separate area to that which you wish to analyse.

Ce reduction as a function of auto-Z
- Samples ought be analysed within the first few hours of introduction to a vacuum chamber. This might mean not taking advantage of modern instruments capacity for large batches of samples and instead recording each material as a separate experiment.
- Take care when determining sample position. If using instrument ‘auto-z’ features to determine sample height, consider performing this on a separate spot at the same dimensional plane and then recording the spectra at a ‘fresh’ point.
- Determine which X-ray power is suitable (if applicable). For ceria-heavy systems with a large signal:noise, using low powered X-ray sources will minimise sample degradation.
- For low ceria content samples, consider using instrument imaging tools to record single scans of multiple areas and aggregate them post-analysis. This will ensure minimal degradation without sacrificing signal intensity.
- Take care when using ancillary techniques, record XPS before (on a separate area) and after (on the analysis area) to determine the impact of your chosen technique on surface chemistry.
Due to the complexity of the 3d emission, at times it may be preferable to analyse the 4d region instead (or if there is significant overlap with a competing peak).
The cerium valence band may also offer insights into cerium oxidation state, given a Ce 4f population for Ce 3+ at around 1.2 eV. This offers a route into speciation by VB XPS and UPS analysis,9 although this becomes a complicated task for mixed oxide systems, or CeOx based composites – due to overlapping emissions in and around the valence region.

Cerium spectra may be assessed qualitatively in pure forms, however mixed oxidation state materials may be deconvoluted successfully and has been done in many pieces of literature.2
Using the Area, FWHM and Position constraints in CasaXPS is vital to obtaining a physically relevant deconvolution of Ce 3d XPS spectra (we would also recommend using the component index feature to organise your peak models).
Use literature values, or better yet use models developed from analysis of pure standard materials on the same XPS instrument as the unknown mixture (to account for spectrometer line width etc), to create a peak model to assess 3+/4+ ratios.
The video below contains a quick guide to approaching the analysis of Ce 3d XPS spectra using the work of Romeo et al.3-5
It is important to use literature values as a guide only, since you may find differences in binding energy, FWHM and even small changes to area ratios as a result of instrument factors (line broadening, X-ray source), experimental factors (calibration) and sample factors (particle size changes can induce lattice strain which can in turn affect binding energies).6
Peaks in Ce4+ 3d analysis are typically labelled u/v (Ce 3d94f2 O 2p4 final state), uII,vII (Ce 3d94f1 O 2p5 final state) and uIII/vIII (Ce 3d94f0 O 2p6 final state).2
Peaks in Ce3+ 3d analysis are typically labelled u0/v0 (Ce 3d94f2 O 2p5 final state) and uI/vI (Ce 3d94f1 O 2p6 final state).2
A table of the initial and final states may be found below (table 1).
For reference, the table of values from the work of Romeo et al3 is below:
| v0 | vI | u0 | uI | v | vII | vIII | u | uII | uIII | |
| Area/Intensity / a.u. | 2.9 | 5.4 | 1.8 | 3.4 | 3.9 | 2.5 | 3.8 | 2.6 | 1.7 | 2.6 |
| FWHM / eV | 0.9 | 1.4 | 0.9 | 1.4 | 0.9 | 1.54 | 0.9 | 1 | 1.53 | 1 |
| Binding energy / eV | 880.6 | 885.4 | 898.9 | 904 | 882.6 | 888.8 | 898.4 | 901 | 907.4 | 916.7 |
Not available
- De Groot, F. and A. Kotani (2008). Core level spectroscopy of solids, CRC press. Find it online here
- Bêche, E., et al. (2008). “Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz).” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 40(3‐4): 264-267. Read it online here
- Romeo, M., et al. (1993). “XPS study of the reduction of cerium dioxide.” Surface and interface analysis 20(6): 508-512. Read it online here
- Paparazzo, E. (2018). “Use and mis-use of x-ray photoemission spectroscopy Ce3d spectra of Ce2O3 and CeO2.” Journal of Physics: Condensed Matter 30(34): 343003. Read it online here
- Paparazzo, E. (2011). “On the curve-fitting of XPS Ce (3d) spectra of cerium oxides.” Materials Research Bulletin 46(2): 323-326. Read it online here
- Deshpande, S., et al. (2005). “Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide.” Applied Physics Letters 87(13): 133113. Read it online here
- Pfau, A. and K. Schierbaum (1994). “The electronic structure of stoichiometric and reduced CeO2 surfaces: an XPS, UPS and HREELS study.” Surface science 321(1-2): 71-80. Read it online here.
- Isaacs, Mark A., et al. “XPS surface analysis of ceria-based materials: Experimental methods and considerations.” Applied Surface Science Advances 18 (2023): 100469.Read it online here.
- Cardenas, Luis, Clément Molinet-Chinaglia, and Stéphane Loridant. “Unraveling Ce 3+ detection at the surface of ceria nanopowders by UPS analysis.” Physical Chemistry Chemical Physics 24.37 (2022): 22815-22822. Read it online here.
Recommended Reading