
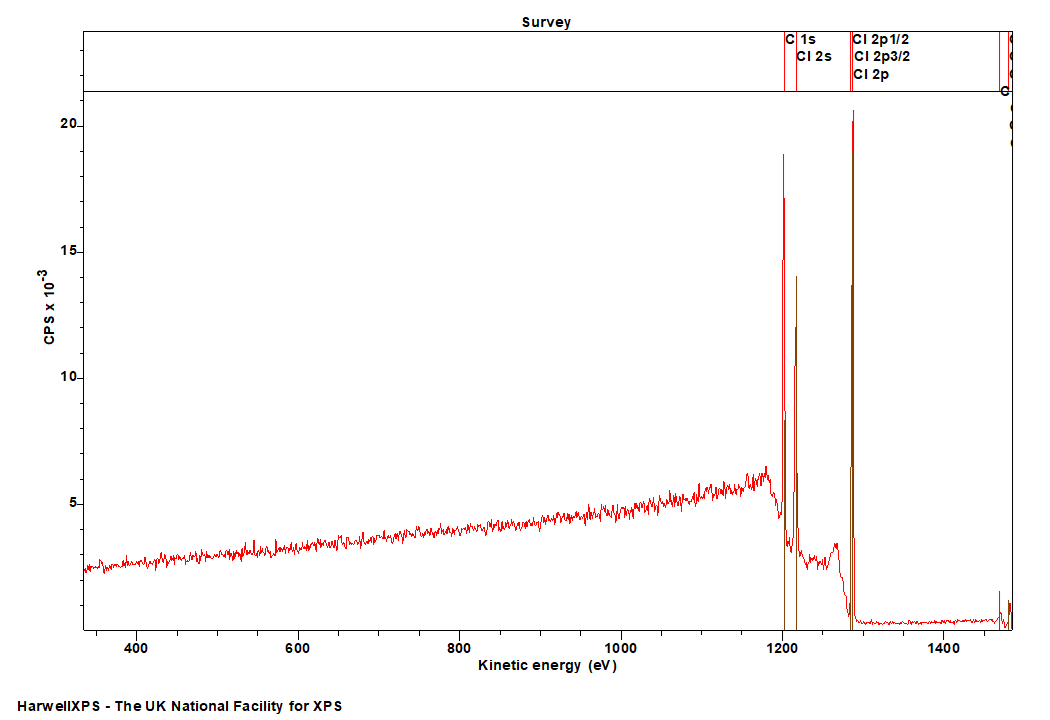
Chlorine
Doublet Separations
- Cl 2p: 1.8 eV
The Energies Listed are Binding Energies!
- Cl 1s: 2830 eV (HAXPES only)
- Cl 2s: 270 eV
- Cl 2p: 190 eV
- Cl 3s: 18 eV
- Cl 3p: 7 eV
The Energies Listed are Binding Energies!
Cl is primarily analysed via the 2p orbital
- Ra 5p: 200 eV
- As 3d: 204 eV
- Nb 3d: 205 eV
- Lu 4d: 205 eV
- La 4p: 206 eV
- Ce 4p: 208 eV
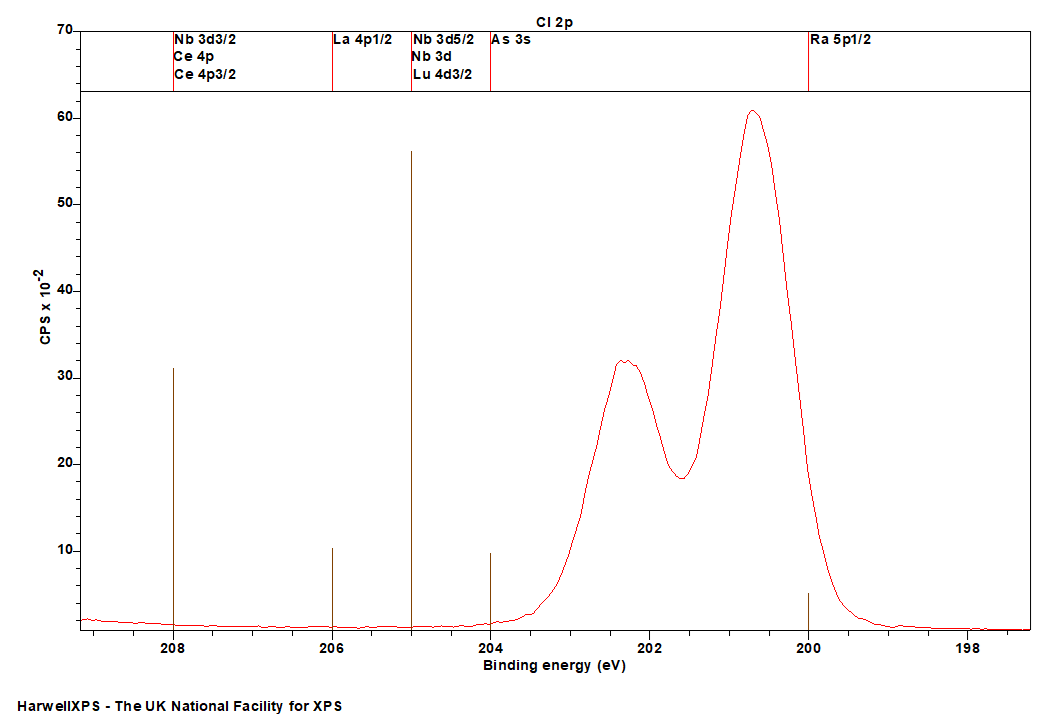
Energies listed are Kinetic Energies!
Cl KLL: ~ 181 eV
The Energies Listed are Binding Energies!
Some reference binding energies for a variety of common chlorine containing compounds can be found in table 1.
| Species | Binding energy / eV | Charge Ref | Ref |
| CH-Cl (PVC) | 200.1 | C 1s (285.1 eV) | 2 |
| NaClO4 | 208.9 | C 1s (284.8 eV) | 3 |
| MgCl2 | 198.5 | C 1s (285 eV) | 4 |
| TiCl4 | 198 | Au 4f (83.8 eV) | 5 |
| AgCl2 | 197.8 | Ag 3d (368.2 eV) / Ag(111) EF | 6 |
| CuCl2 | 198.9 | C 1s (284.6 eV) | 7 |
| CuCl | 198.8 | C 1s (284.5 eV) | 8 |
| SrCl2 | 198.9 | C 1s (284.6 eV) | 9 |
| PdCl2 | 198.9 | C 1s (284.8 eV) | 10 |
| NaCl | 198.7 | C 1s (284.8 eV) | 11 |
| NaClO3 | 206.4 | C 1s (284.8 eV) | 12 |
| NaClO2 | 204.7 | C 1s (284.8 eV) | 13 |
Chlorine XPS analysis very straightforward and is typically performed on the 2p region where overlaps are restricted to the Se LMM auger and As 3s photoemission.
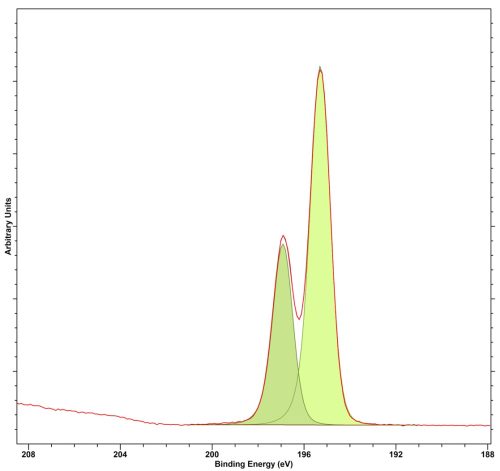
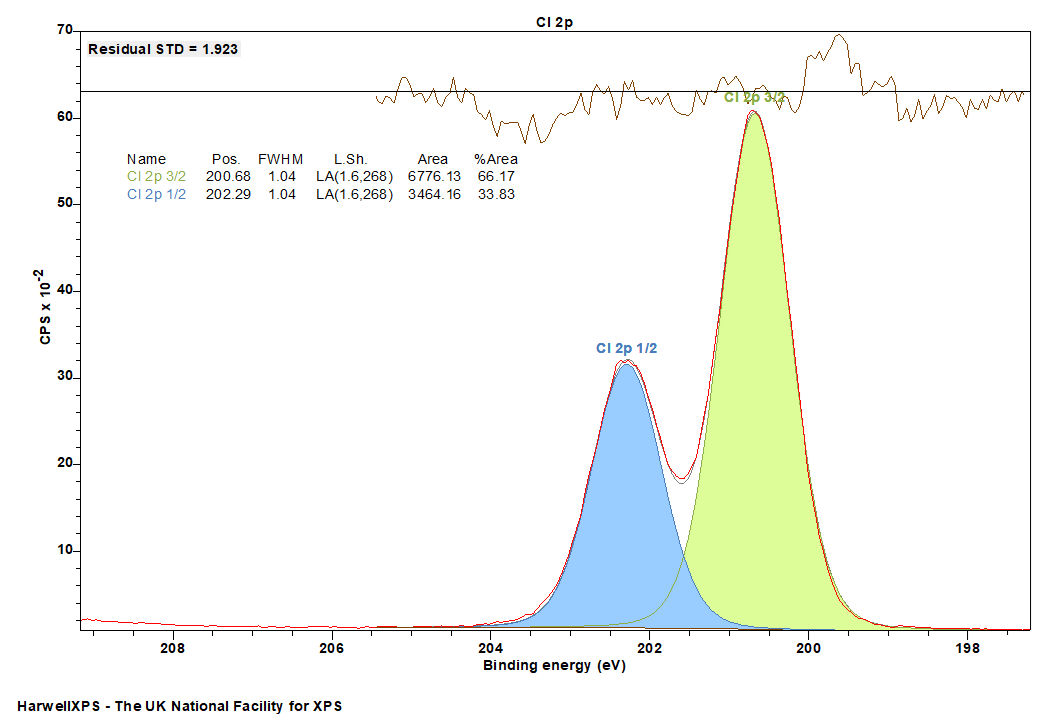
Cl 2p peaks have a doublet separation of 1.6 eV.
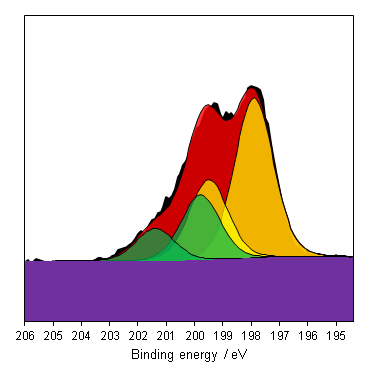
Chlorine is a common contaminant in XPS, typically from NaCl salts from sweat on poorly handled samples. Always wear gloves when handling XPS samples!
Chlorine polymers have been observed to degrade under continual X-ray exposure, be sure to take a quick scan before and after full measurement to check for degradation of an new or unknown sample.[14]
Cl 2p is easily fit with a typical Voight-type lineshape.
Not available
Not available
- Spectrum recorded by HarwellXPS
- Lannon Jr, J. M. and Q. Meng (1999). “Analysis of a Filled Poly (vinyl chloride) Polymer by XPS.” Surface Science Spectra 6(2): 131-136. Read it online here.
- Beard, B. C. (1993). “Sodium salts of chlorine oxyacid anions, Cl (+ 7), perchlorate, XPS comparison spectra.” Surface Science Spectra 2(2): 97-103. Read it online here.
- Garbassi, F. and L. Pozzi (1979). “Electron beam effects on hydrated magnesium chloride revealed by XPS.” Journal of Electron Spectroscopy and Related Phenomena 16(2): 199-203. Read it online here.
- Mousty‐Desbuquoit, C., et al. (1983). “Solid state effects in the electronic structure of TiCl4 studied by XPS.” The Journal of chemical physics 79(1): 26-32. Read it online here.
- Piao, H., et al. (2004). “A temperature-programmed X-ray photoelectron spectroscopy (TPXPS) study of chlorine adsorption and diffusion on Ag (1 1 1).” Surface science 557(1-3): 13-20. Read it online here.
- Vasquez, R. P. (1993). “CuCl2 by XPS.” Surface Science Spectra 2(2): 160-164. Read it online here.
- Vasquez, R. P. (1993). “CuCl by XPS.” Surface Science Spectra 2(2): 138-143. Read it online here.
- Vasquez, R. P. (1992). “SrCl2 by XPS.” Surface Science Spectra 1(1): 68-74. Read it online here.
- Militello, M. C. and S. J. Simko (1994). “Palladium chloride (PdCl2) by XPS.” Surface Science Spectra 3(4): 402-409. Read it online here.
- Beard, B. C. (1993). “Fresh cleaved single crystal NaCl, XPS spectra, Al source.” Surface Science Spectra 2(2): 91-96. Read it online here.
- Beard, B. C. (1993). “Sodium Salts of Chlorine Oxyacid Anions, Cl (+ 5), Chlorate, XPS Comparison Spectra.” Surface Science Spectra 2(1): 26-30. Read it online here.
- Beard, B. C. (1993). “Sodium Salts of Chlorine Oxyacid Anions, Cl (+ 3), Chlorite, XPS Comparison Spectra.” Surface Science Spectra 2(1): 20-25. Read it online here.
- Morgan, David J., and Sharukaa Uthayasekaran. “Revisiting degradation in the XPS analysis of polymers.” Surface and Interface Analysis 55.6-7 (2023): 556-563. Read it online here.