
Lithium
Doublet Separations
- No non-S-orbital emissions
The Energies Listed are Binding Energies!
- Li 1s: 55 eV
The Energies Listed are Binding Energies!
Overlaps for Li 1s (primary emission)
- Ir 5p (51 eV)
- Pt 5p (51 eV)
- Ho 5s (51 eV)
- Mg 2p (52 eV)
- Zr 4s (52 eV)
- Os 4f (52 eV)
- Yb 5s (53 eV)
- Tm 5s (53 eV)
- Au 5p (54 eV)
- Sc 3s (54 eV)
- Fe 3p (56 eV)
- Ag 4p (56 eV)
- Pd 4p (56 eV)
- Lu 5s (57 eV)
- S 3d (57 eV)
Energies listed are Kinetic Energies!
- Li KLL: ~ 40 eV
XPS of lithium is performed on the only available core level – the 1s. Since these photoemissions consist of singular peaks, and the sensitivity of XPS towards Li is very low, Li can be very difficult to analyse by XPS.
Li 1s may overlap with a number of photoemissions, such as; Ag 4p, Au 5p, Fe 3p, Pd 4p, Sc 3s, Se 3d, Mg 2p, Zr 4s, Ir 5p, Pt 5p, I 4d which further complicates the determination of Li content and speciation in mixed materials. As a result of this it is highly advised to use long scan times when probing Li 1s regions.
Furthermore, the highly reactive nature of lithium means inert sample transfer is required to load samples within a glovebox prior to transportation to the XPS system. Shown below is one of the multiple inert sample handling options available at the HarwellXPS Hub.

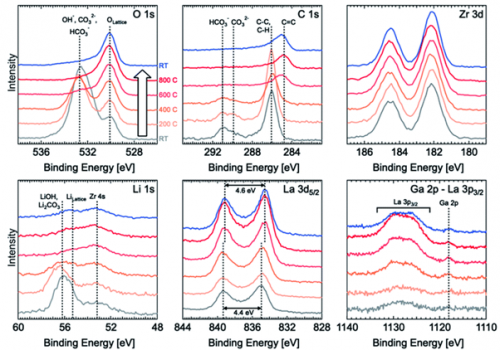
One school of thought suggests that the use of binding energy separations between Li and O emissions provides a more useful tool for the determination of chemical species (which doesn’t require an unreliable carbon energy correction).(1) This method may fall down in the analysis of common mixed oxide systems such as LLZO (Lithium Lanthanum Zirconium Oxide), however in such a case the use of the lattice oxygen at 530.1 eV as an energy correction factor has been reported to be very effective (Figure 1).(2)
Not available
Not available
References
- Hoenigman, J. R., and R. G. Keil. “An XPS study of the adsorption of oxygen and water vapor on clean lithium films.” Applications of surface science 18.1-2 (1984): 207-222. Read it online here.
- Contarini, Salvatore, and J. Wayne Rabalais. “Ion bombardment-induced decomposition of Li and Ba sulfates and carbonates studied by X-ray photoelectron spectroscopy.” Journal of electron spectroscopy and related phenomena 35.2 (1985): 191-201. Read it online here.
- Wood, K. N. and G. Teeter (2018). “XPS on Li-battery-related compounds: analysis of inorganic SEI phases and a methodology for charge correction.” ACS Applied Energy Materials 1(9): 4493-4504. Read it online here.
- Brugge, R., et al. (2020). “The Origin of Chemical Inhomogeneity in Garnet Electrolytes and its Impact on the Electrochemical Performance.” Journal of Materials Chemistry A. Read it online here.