
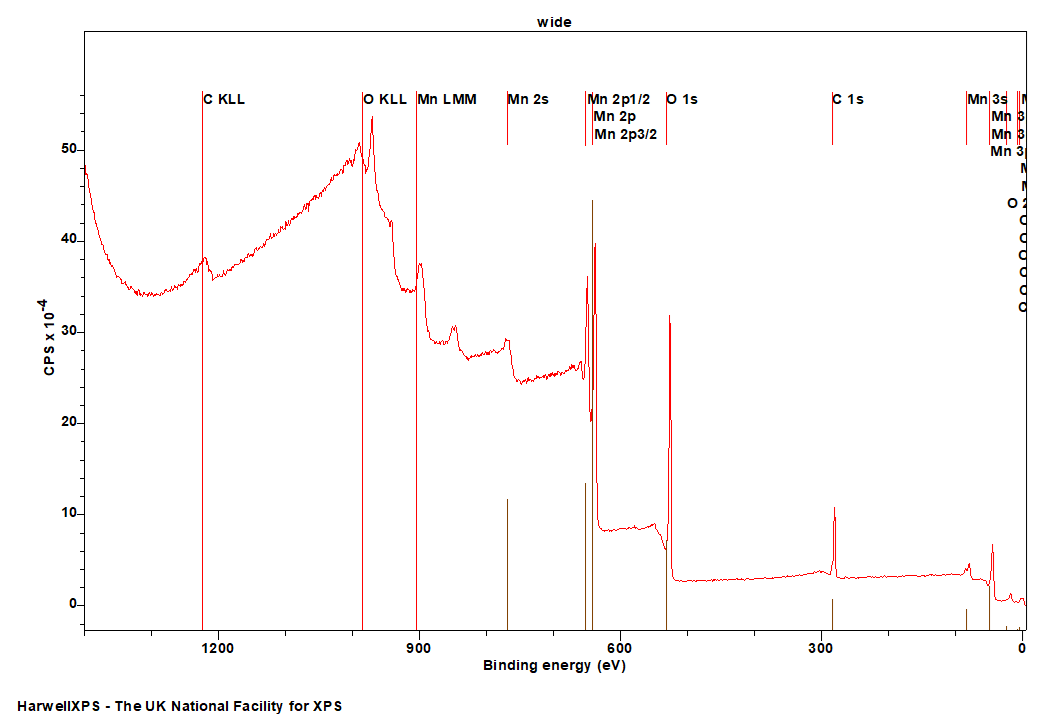
Manganese
Doublet Separations
- Mn 2p: 11.2 eV (metal)
- Mn 2p: 11.7 eV (oxides)
- Mn 3s: Variable – see experimental section below
The Energies Listed are Binding Energies!
- Mn 2s: 769 eV
- Mn 2p: 641 eV
- Mn 3s: 84 eV
- Mn 3p: 49 eV
- Mn 3d: 4 eV
The Energies Listed are Binding Energies!
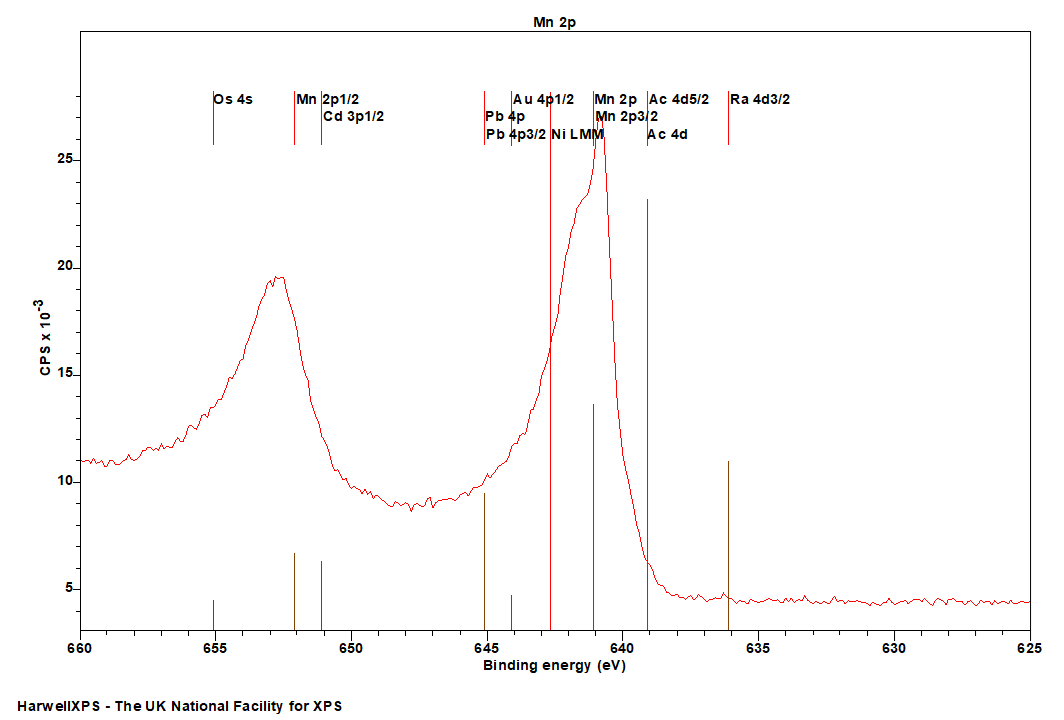
Sb is primarily analyzed via the 3d orbital
- Ra 4d (636 eV)
- Ac 4d (639 eV)
- Ni LMM (Al source) (~ 642 eV)
- Au 4p (644 eV)
- Pb 4p (645 eV)
- Cd 3p (651 eV)
- Os 4p (655 eV)
- In 3p (664 eV)
Energies listed are Kinetic Energies!
Mn MNN: ~ 580 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| Mn(0) | 638.6 | Au 4f (83.95 eV) | 1 |
| Mn(II) | 639.3 | Au 4f (83.96 eV) | 2 |
| Mn(III) | 640.8 | Au 4f (83.96 eV) | 2 |
| Mn(IV) | 642 | Au 4f (83.96 eV) | 2 |
It should be noted, that binding energies alone are not of great use when trying to understand Mn XPS – see the sections below for more information
Manganese speciation using the 2p region can be quite difficult due to complex multiplet splitting, and subtle differences in the binding energy of the various emissions.
Often recording the Mn 2p region alone may not be the most practical approach, given the complexities of multiplet splitting in Manganese.
Recording both the Mn 2p, and the Mn 3p provides opportunity for two models in order to confirm the speciation (using the models of Ilton)[2], and if possible, the Mn 3s also. It may not always be possible, given the lower cross-sections of the 3p and 3s orbitals compared to the 2p, and if the sample is low in Mn content these regions may not provide enough signal intensity for accurate modelling.
It is common practice to record the Mn 3s peak in order to speciate based on the splitting energy – however Ilton et al report that this is not always reliable when considering mixed oxide systems.[2]
Modelling the Mn 2p is a possibility, and through the systems implemented by Ilton and Biesinger,[1] peak models may be developed that can describe the distinct states within select Mn compounds. Modelling the 3p region using the parameters provided int he work of Ilton, or using standard datasets, provides a second check for the sample which may be advantageous given the complex peak fits involved.
Care should be taken in the interpretation of these datasets, given the vast difference in kinetic energies of the Mn 2p and 3p photoelectrons – and if the samples is inhomogeneous in the z-dimension then this process will not be comparable.
Not available
Not available
- Biesinger, Mark C., et al. “Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni.” Applied Surface Science 257.7 (2011): 2717-2730. Read it online here.
- Ilton, Eugene S., et al. “XPS determination of Mn oxidation states in Mn (hydr) oxides.” Applied Surface Science 366 (2016): 475-485. Read it online here.
