
Niobium
Doublet Separations
- Nb 3d: 2.78 eV
- Nb 3p: 15.5 eV
- Nb 4p: 1.8 eV
The Energies Listed are Binding Energies!
- Nb 3s: 469 eV
- Nb 3p: 363 eV
- Nb 3d: 205 eV
- Nb 4s: 58 eV
- Nb 4p: 34 eV
- Nb 4d: 4 eV
The Energies Listed are Binding Energies!
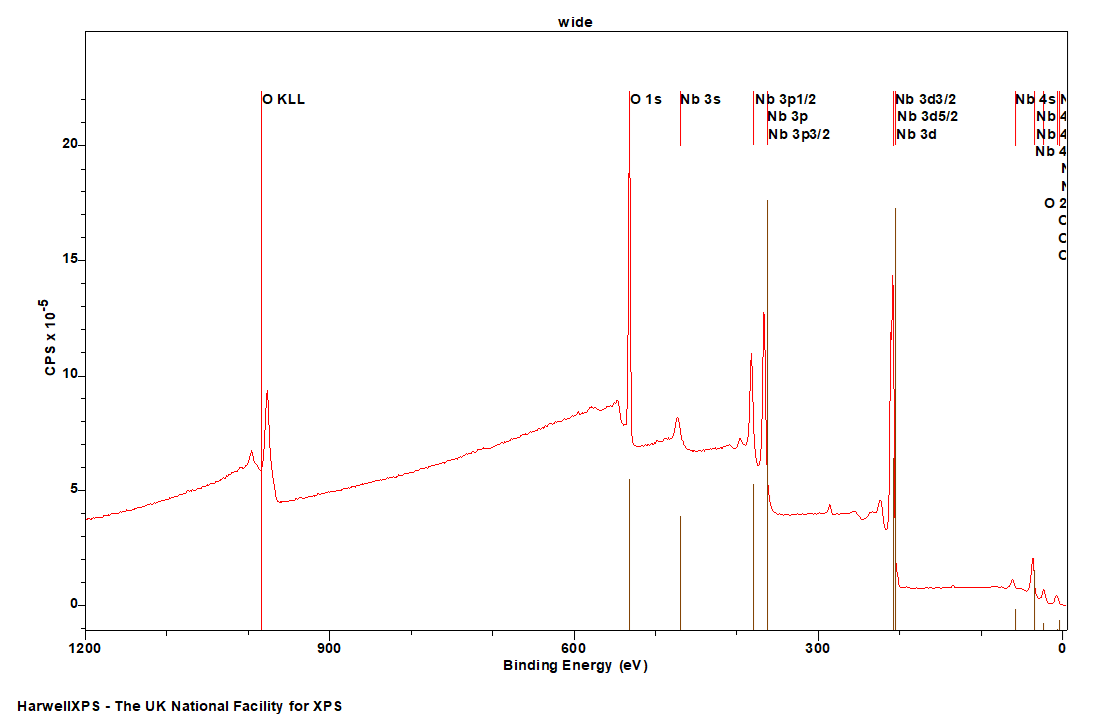
Nb is primarily analysed via the 3d orbital
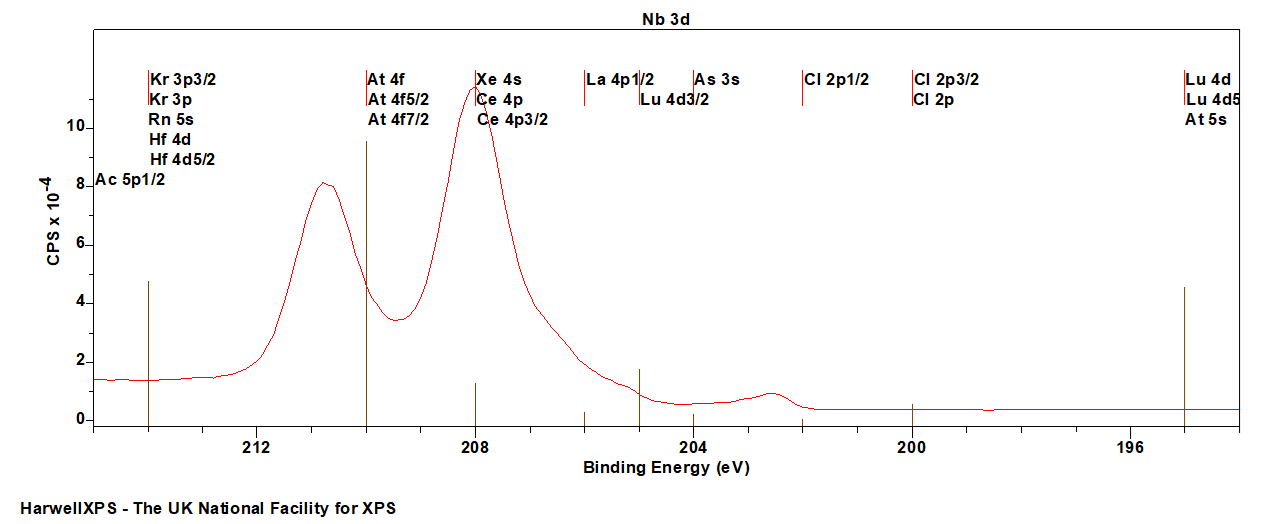
- Cl 2p (200 eV)
- As 3s (204 eV)
- Lu 4d (205 eV)
- La 4p (206 eV)
- Ce 4p (208 eV)
- Xe 4s (208 eV)
- At 4f (210 eV)
- Kr 3p (214 eV)
- Rn 5s (214 eV)
- Hf 4d (214 eV)
- Ac 5p (215 eV)
Energies listed are Kinetic Energies!
Nb MNV: ~ 168 eV
Nb LMM (Ag anode): ~ 1940 eV
The Energies Listed are Binding Energies!
Niobium (Nb) is a transition metal known for its exceptional properties, making it important in various industrial and scientific applications. It is widely used in alloy production, especially in superalloys, where it enhances strength, corrosion resistance, and heat resistance. Niobium is commonly found in the production of stainless steel, titanium alloys, and aerospace components. Its ability to form stable niobium oxide (Nb₂O₅) also makes it valuable in electronic capacitors, particularly in high-performance capacitors used in telecommunications, computing, and automotive systems. Niobium is also utilized in superconducting magnets for medical imaging (MRI) and particle accelerators, owing to its superconducting properties when cooled to low temperatures. Additionally, niobium plays a key role in pharmaceuticals and nuclear applications, where it is used as a corrosion-resistant material.
Cl 2p can be a significant overlap with Nb 3d, though this is primarily problematic for Nb metal, the shift in Nb oxides should enable facile deconvolution of any overlap, though care should be taken to record a large enough region to apply suitable backgrounds during analysis.
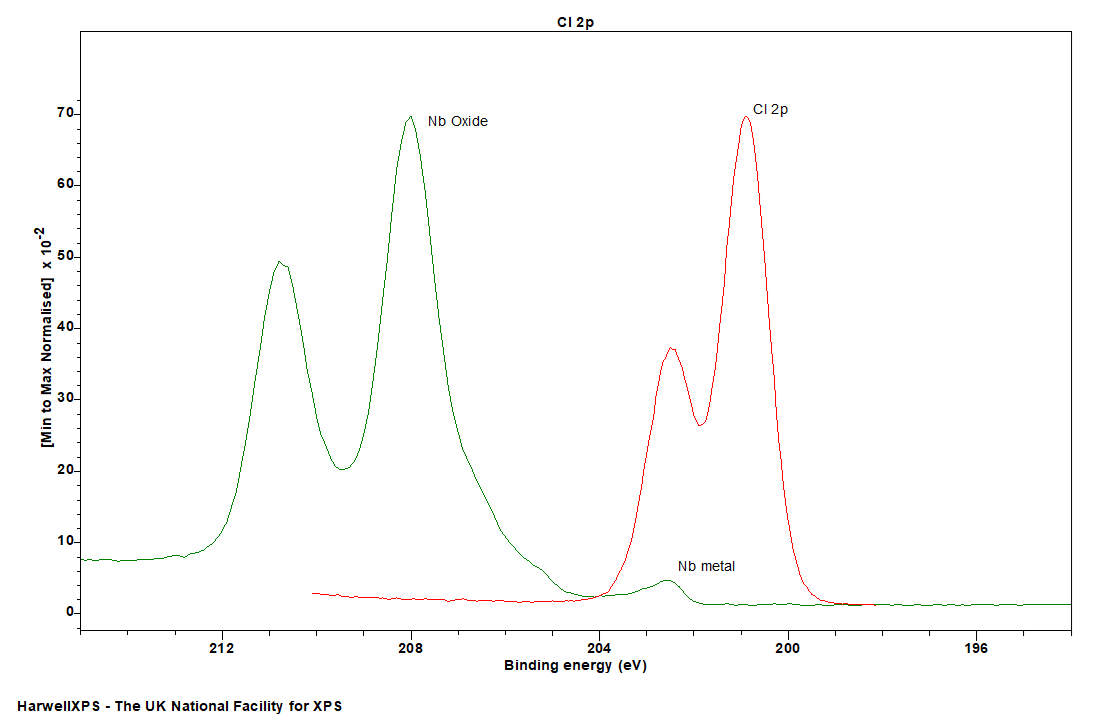
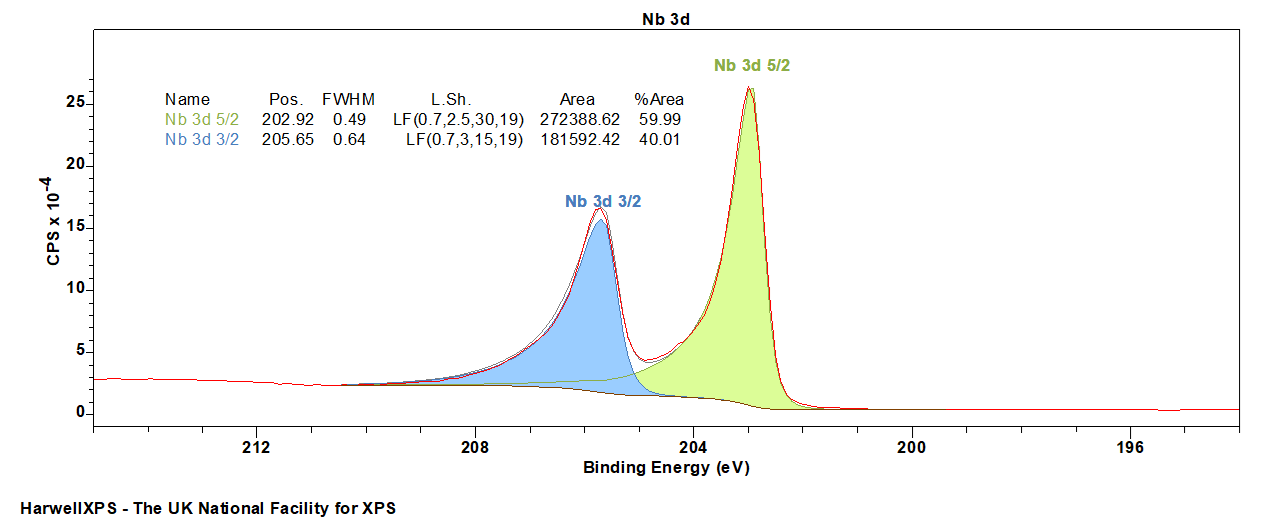
Niobium metal exhibits asymmetric peaks, and slightly broadened Nb 3d 3/2 peaks due to Coster-Kronig broadening.
Not available
Not available
- Powell, C. J. “Elemental binding energies for X-ray photoelectron spectroscopy.” Applied Surface Science 89.2 (1995): 141-149. Read it online here.
- Gomes, Maria AB, et al. “The electrochromic process at Nb2 O 5 electrodes prepared by thermal oxidation of niobium.” Journal of the Electrochemical Society 137.10 (1990): 3067. Read it online here.
- Bahl, M. K. “ESCA studies of some niobium compounds.” Journal of Physics and Chemistry of Solids 36.6 (1975): 485-491. Read it online here.