Oxygen (Organics)
Doublet Separations
- No relevant non-S-orbital emissions
The Energies Listed are Binding Energies!
- O 1s: 530 eV
- O 2s: 20 eV
- O 2p: 8 eV
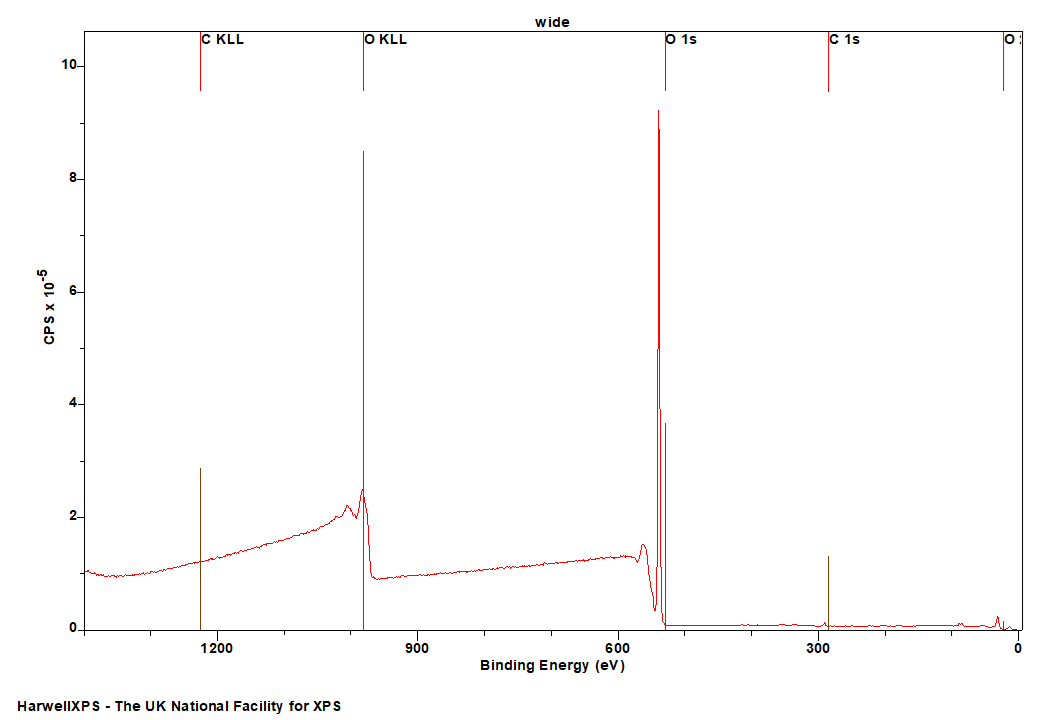
The Energies Listed are Binding Energies!
Overlaps for O 1s (primary emission)
- Na KLL (Al kα X-rays) (500 eV)
- V 2p (512 eV)
- Dy MNN (Al kα X-rays) (517 eV)
- Sb 3d (528 eV)
- Pd 3d (531 eV)
- At 4d (533 eV)
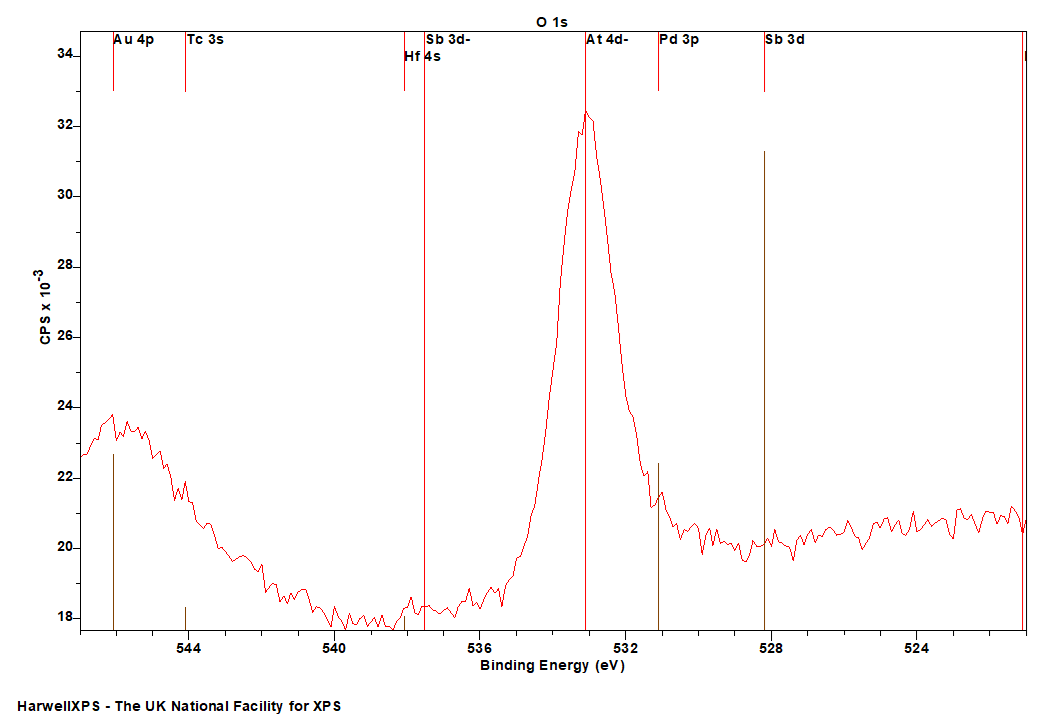
Energies listed are Kinetic Energies!
O KLL: ~ 505 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| C – O – C (Aliphatic) | 532.6 ± 0.2 | C (285 eV) | Ref |
| C – O – C (Aromatic) | 533.25 ± 0.2 | C (285 eV) | Ref |
| C – O – H (Aliphatic) | 532.9 ± 0.1 | C (285 eV) | Ref |
| C – O – H (Aromatic) | 533.6 | C (285 eV) | Ref |
| C = O (Aliphatic) | 532.3 | C (285 eV) | Ref |
| C = O (Aromatic) | 531.2 | C (285 eV) | Ref |
| Ph – O – Ph | 533.3 | C (284.7 eV) | Ref |
| O – C – O | 533.15 ± 0.2 | C (285 eV) | Ref |
| O – C = O | 532.3 | C (285 eV) | Ref |
| O – C = O | 533.9 | C (285 eV) | Ref |
| – C – O – C – || || O O |
532.6 ± 0.2 | C (285 eV) | Ref |
| – C – O – C – || || O O |
533.9 ± 0.1 | C (285 eV) | Ref |
| O – C – O || O |
532.4 ± 0.1 | C (285 eV) | Ref |
| O – C – O || O |
533.9 ± 0.05 | C (285 eV) | Ref |
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| CF2 – O CF2 | 532.3 | C (285 eV) | Ref |
| O – C(O) – CF3 | 533.8 | C (285 eV) | Ref |
| O – C(O) – CF3 | 532.6 | C (285 eV) | Ref |
| – SO2 – | 531.8 ± 0.1 | C (285 eV) | Ref |
| Ph – S – Ph | 531.6 | C 1s (284.7) | Ref |
| – SO3 | 531.7 | C (285 eV) | Ref |
| Ph-SO3Na | 531.7 | C (285 eV) | Ref |
| C – O – P | 533.4 | C (285 eV) | Ref |
| – PO2 | 533.2 | P 2p (135 eV) | Ref |
| – PO4 | 532.75 ± 0.15 | P 2p (135 eV) | Ref |
| –OPO4 | 533.1 | P 2p (135 eV) | Ref |
| –OHPO4 | 532.6 | P 2p (135 eV) | Ref |
| P2O7 | 532.35 | P 2p (135 eV) | Ref |
| P3O10 | 532.2 | P 2p (135 eV) | Ref |
| P4O12 | 531.8 | P 2p (135 eV) | Ref |
| P2O5 | 533.5 | P 2p (135 eV) | Ref |
The O 1s core level is particularly important in XPS analysis because it provides detailed information about the chemical states of oxygen atoms within the material. In polymers and organics, the O 1s peak can reveal the presence of various functional groups such as hydroxyls, carbonyls, and ethers, which are crucial for understanding the material’s properties and behavior. The binding energy of the O 1s electrons varies depending on the specific chemical environment, allowing for the identification and quantification of these functional groups. Accurate interpretation of O 1s XPS spectra requires careful consideration of factors such as sample preparation, charge neutralization, and data processing to ensure reliable results.
Analysis of polymers by XPS may lead to sample damage and degradation which tends to be led by a loss of oxygen, and as such O 1s should be taken as the initial scan – though it is worth analysing a number of areas with different analysis orders to assess damage.
Sample damage for ethers tends to decrease with increasing length of side chains.[1]
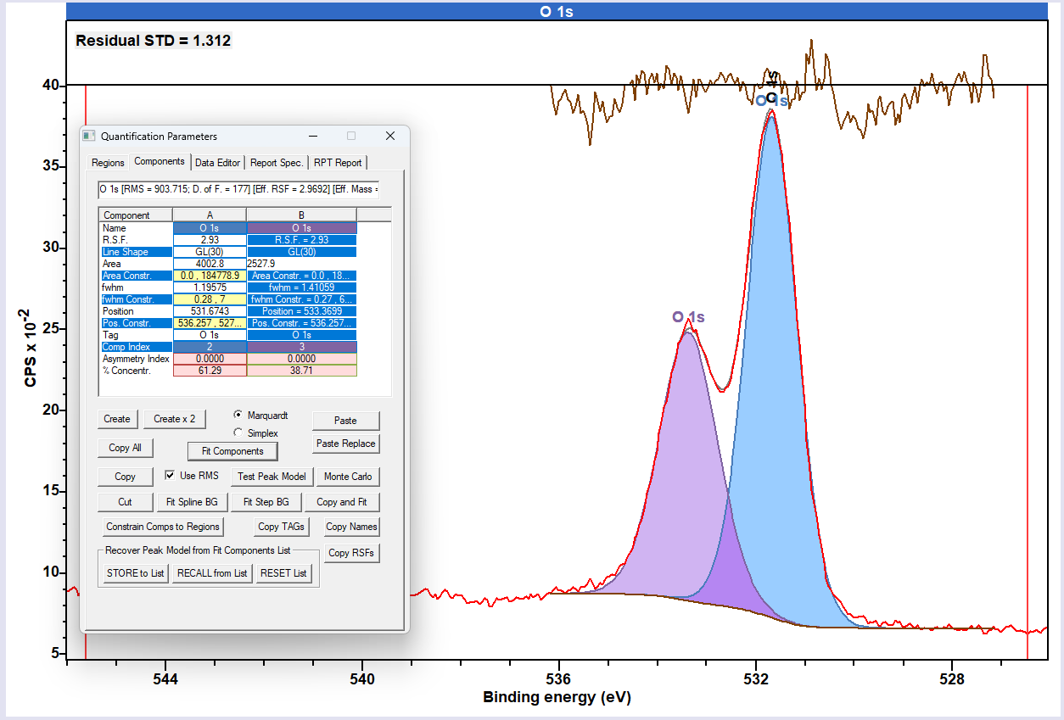
O 1s peaks in polymers tend to be slightly more Lorentzian in nature than C 1s, and the classic GL(30) lineshape may not always provide the best fit. While it is possible to fit using a modified GL (e.g. GL50), one often finds it better to use the LA type lineshape for improved fits.
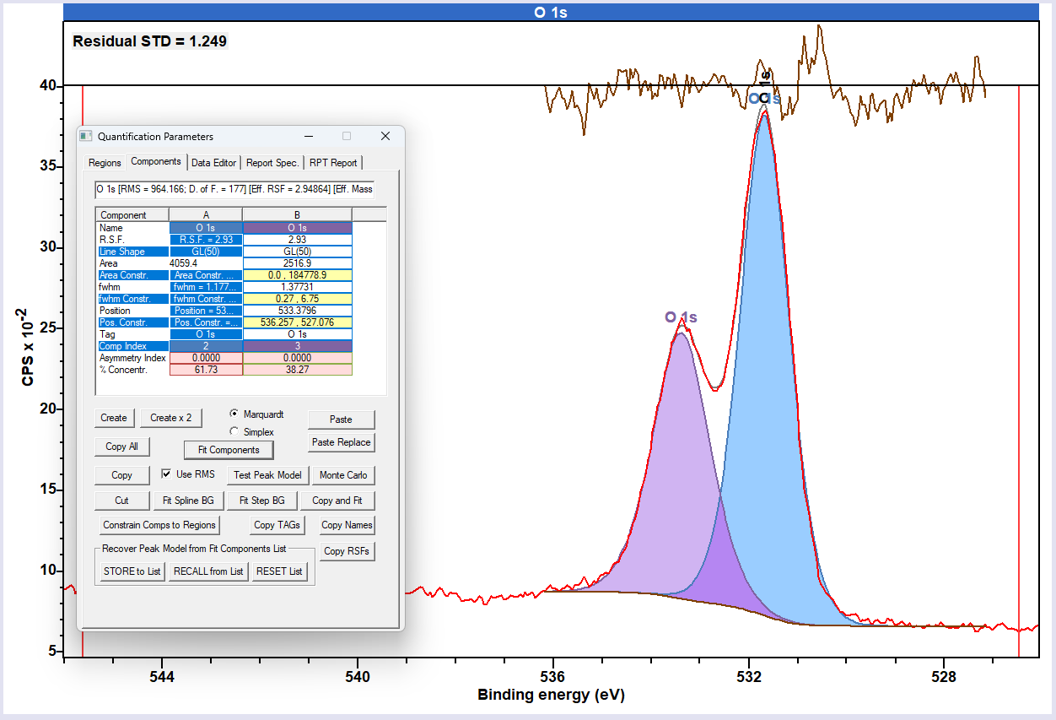
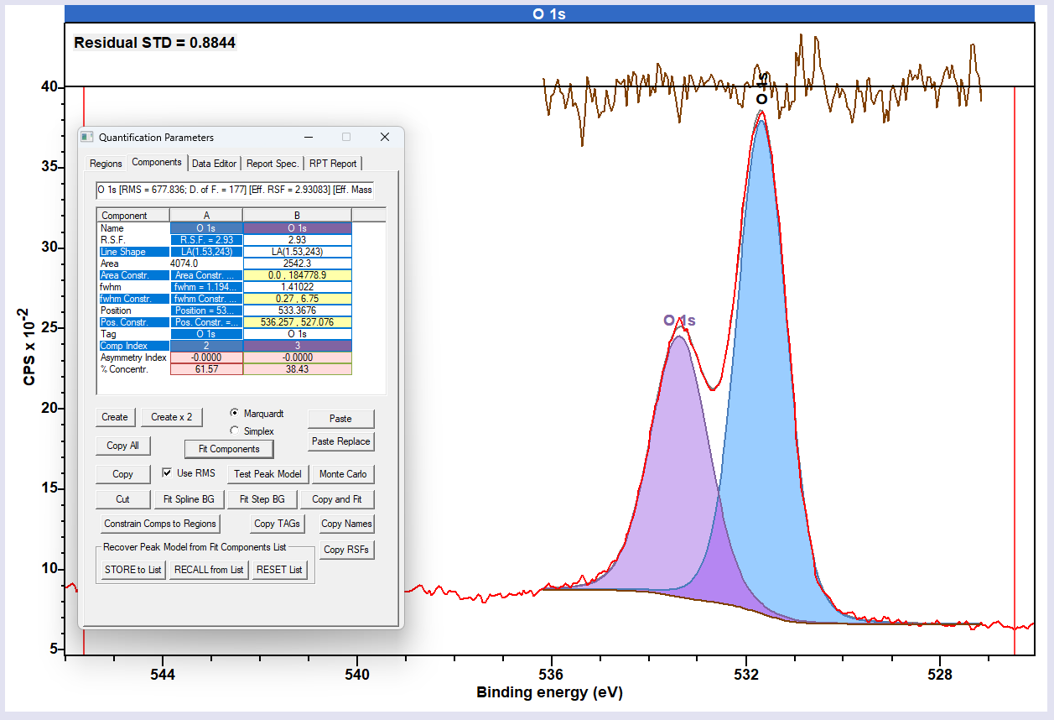
Not available
Not available
References
- Hantsche, H. (1993), High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., hardcover, £ 65.00, ISBN 0-471-93592-1. Adv. Mater., 5: 778-778. https://doi.org/10.1002/adma.19930051035