
Palladium
Doublet Separations
- Pd 3d: 5.25 eV
- Pd 3p: 27.6 eV
The Energies Listed are Binding Energies!
- Pd 3s: 670 eV
- Pd 5p: 531 eV
- Pd 3d: 334 eV
- Pd 4s: 86 eV
- Pd 4p: 41 eV
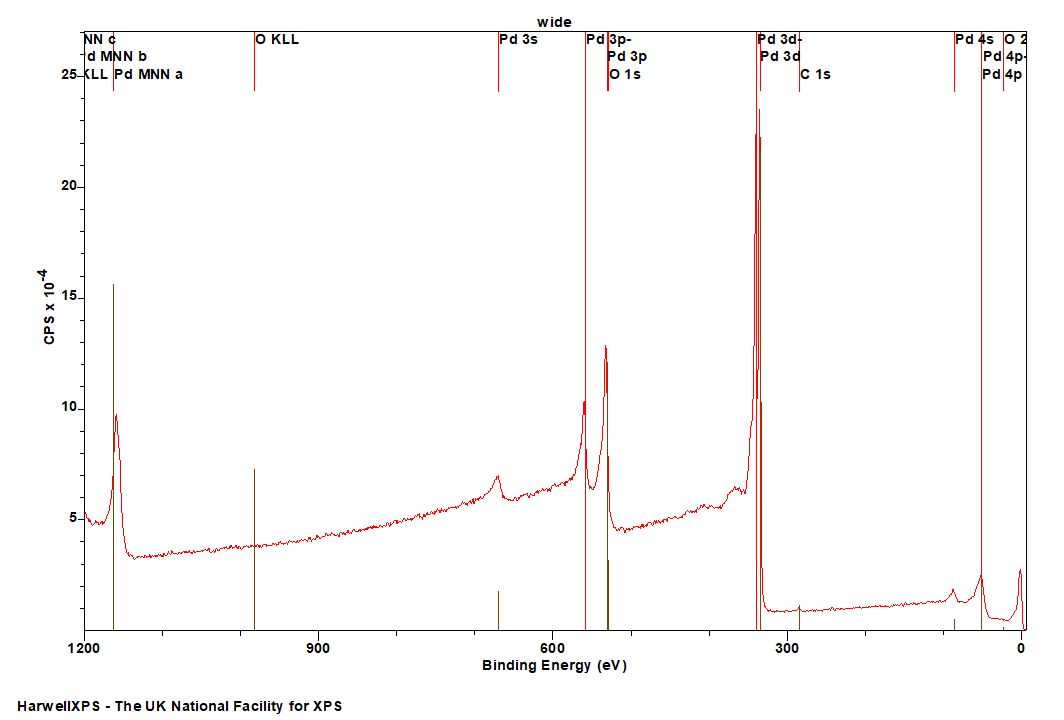
The Energies Listed are Binding Energies!
Pd is primarily analysed via the 3d orbital
- Dy MNN b (Al source) (517 eV)
- Re 4p1/2 (518 eV)
- Pt 4p3/2 (519 eV)
- V 2p1/2 (519.8 eV)
- Rh 3p1/2 (521 eV)
- O 1s (529.1 eV)
- Pd 3p3/2 (531 eV)
- Hf 4s (538 eV)
- Au 4p3/2 (546 eV)

Energies listed are Kinetic Energies!
Pd MNN: ~ 324 eV
Pd NOO: ~ 40 eV
The Energies Listed are Binding Energies!
| Species | 3d5/2 Binding energy / eV | Ref |
| Pd metal | 334.8-335.5 (Typically taken as 335.2)(4) | 5,6,7,8 |
| PdO | 336.3-337.2 | 8,9 |
| PdCl2 | 338.4 (Pd0 = 335.2) | 10 |
| PdS2 | 336.6 (Pd0 = 335.5) | 11 |
| Na2PdCl4 | 337.9 (Pd0 = 335.5) | 11 |
| K2PdCl4 | 338.1 (Pd0 = 335.5) | 11 |
Palladium is a fantastic catalyst for many organic transformations, for example selective oxidation, and as such understanding the surface chemistry is vital and has been extensively studied.
Palladium is typically analysed using the Pd 3d region, which does not have any particularly unusual characteristics to be aware of.
Pd2+ species have the capability to reduce under an X-ray beam and as such should ideally be the first region analysed during a measurement.(1)
The most significant overlapping spectral features are the 3p peaks from zirconium, the 4d region of gold and some secondary auger features from the Mg KLL auger when using an aluminium X-ray source (1486.7 eV), so take care when analysing mixtures of these, you may potentially need to look at another Pd orbital (although this is complicated due to an overlap between Pd 3p3/2 and O 1s, leaving only the relatively weak Pd 3p1/2 and Pd 3s available for the majority of samples).
Typically, the 3d region is the most useful and widely studied. It has a doublet separation of 5.26 eV and a standard 3d5/2 : 3d3/2 area ratio of 3:2. There is a significant energy separation between Pd metal and PdO (1.7 eV)(2) which facilitates trivial deconvolution of the two species.
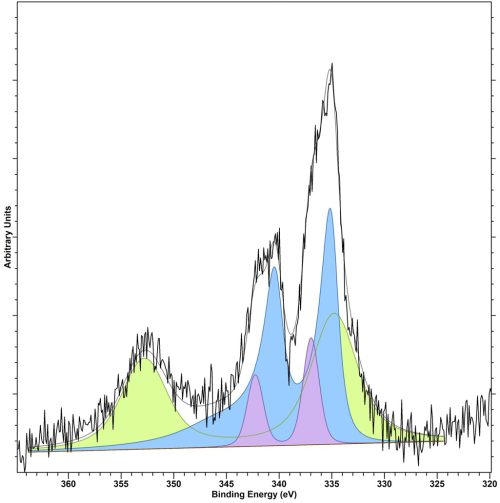
Figure . Fitted Pd(3d)/Au(4d) region for a AuPd/TiO2 catalysts for hydrogen peroxide formation, where: Blue = Pd(0), Lilac = Pd(2+) and Green = Au(0)
If it is possible to accurately obtain, the modified auger parameter (M4,5N1N2,3 & Pd 3d5/2) may provide a more reliable measure of the electronic state of the palladium, although the M4,5N1N2,3 auger is surprisingly weak from Pd metal.(12)
The modified auger parameter (M4,5N1N2,3) for Pd metal is 242.8±0.2 eV, and for PdO is 240.5±0.2.(12) If considering the M4,5VV auger, the parameter for Pd metal becomes 327.5±0.2 eV, while difficulties over the determination of a precise peak maxima render this choice of auger less desirable for PdO.(12)
Not available
Not available
References
- Narayana, M., et al. (1985). “Determination of the chemical state of palladium in PdNa-X zeolite by electron spin resonance and x-ray photoelectron spectroscopy.” The Journal of Physical Chemistry 89(18): 3895-3899. Read it online here.
- Peuckert, M. (1985). “XPS study on surface and bulk palladium oxide, its thermal stability, and a comparison with other noble metal oxides.” The Journal of Physical Chemistry 89(12): 2481-2486. Read it online here.
- Lee, A. F. and K. Wilson (2004). “Structure–reactivity correlations in the selective aerobic oxidation of cinnamyl alcohol: in situ XAFS.” Green chemistry 6(1): 37-42. Read it online here.
- Smirnov, M. Y., et al. (2018). “An XPS Study of the Interaction of a Palladium Foil with NO2.” Kinetics and Catalysis 59(6): 786-791. Read it online here.
- Militello, M. C. and S. J. Simko (1994). “Elemental palladium by XPS.” Surface Science Spectra 3(4): 387-394. Read it online here.
- Briggs, D. (1990). “Practical Surface Analysis second edition Vol. 1, Auger and X-ray Photoelectron Spectroscopy.” John Wiley & Sons Ltd., New York. Find it online here.
- Moulder, J. F. (1995). “Handbook of X-ray photoelectron spectroscopy.” Physical electronics: 230-232. Find it online here.
- Kim, K. S., et al. (1974). “X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode.” Analytical Chemistry 46(2): 197-200. Read it online here.
- Militello, M. C. and S. J. Simko (1994). “Palladium oxide (PdO) by XPS.” Surface Science Spectra 3(4): 395-401. Read it online here.
- Militello, M. C. and S. J. Simko (1994). “Palladium chloride (PdCl2) by XPS.” Surface Science Spectra 3(4): 402-409. Read it online here.
- Hyland, M. and G. Bancroft (1990). “Palladium sorption and reduction on sulphide mineral surfaces: An XPS and AES study.” Geochimica et Cosmochimica Acta 54(1): 117-130. Read it online here.
- Brun, M., et al. (1999). “XPS, AES and Auger parameter of Pd and PdO.” Journal of Electron Spectroscopy and Related Phenomena 104(1-3): 55-60. Read it online here.