- Elements
Phosphorus
References
- Data acquired by HarwellXPS
- Edmonds, M. T., et al. (2015). “Creating a stable oxide at the surface of black phosphorus.” ACS applied materials & interfaces 7(27): 14557-14562. Read it online here.
- Wang, Y. and P. M. Sherwood (2002). “Phosphorus pentoxide (P2O5) by XPS.” Surface Science Spectra 9(1): 159-165. Read it online here.
- Liu, K., et al. (2004). “Surface analysis of (NH2) 2CS‐treated GaP (001) by AES and XPS.” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 36(8): 966-968. Read it online here.

Phosphorous
Doublet Separations
- P 2p: 0.87 eV
The Energies Listed are Binding Energies!
- P 2s: 189 eV
- P 2p: 135 eV
- P 3s: 16 eV
- P 3p: 10 eV
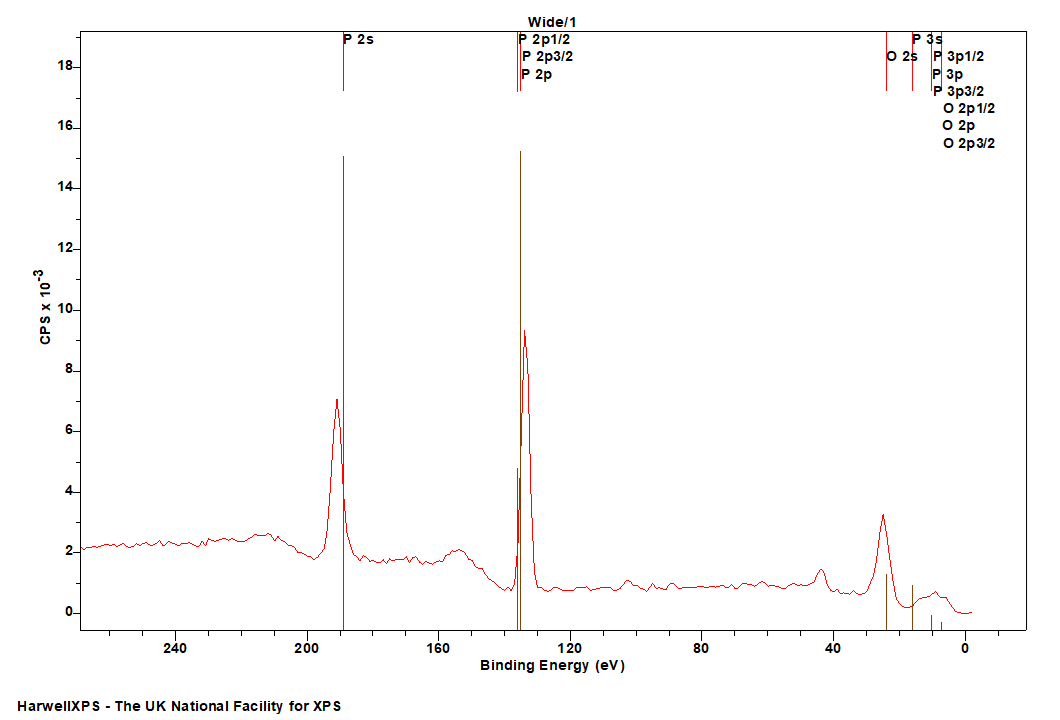
The Energies Listed are Binding Energies!
P is primarily analysed via the 2p orbital
- Ge 3p (129 eV)
- Sm 4d (130 eV)
- Po 5p (132 eV)
- Sr 3d (133 eV)
- Eu 4d (134 eV)
- Sn 4s (137 eV)
- Zn 4s (137 eV)
- Tl 5s (137 eV)
- Pb 4f (138 eV)

Energies listed are Kinetic Energies!
P LMM: ~ 120 eV
The Energies Listed are Binding Energies!
There exists a relatively sizable energy separation between phosphorous states of varying oxidation, permitting relatively trivial deconvolution. Table 1 presents a range of binding energies for common phosphorous species.
Phosphorus is essential in materials science due to its role in semiconductors, biomaterials, and functional coatings. It is a key component in phosphorene, a two-dimensional material with promising electronic and optoelectronic applications, and phosphate-based ceramics, which are widely used in biomedical implants and catalysis. Phosphorus is also crucial in LiFePO₄ battery materials, flame retardants, and corrosion-resistant coatings.
XPS analysis of phosphorous is typically performed on the P 2p region which may overlap with Sr 3d peaks or with the Zn 3s peak if the phosphorous has a high oxidation state. The doublet separation of P 2p is 0.86 eV.
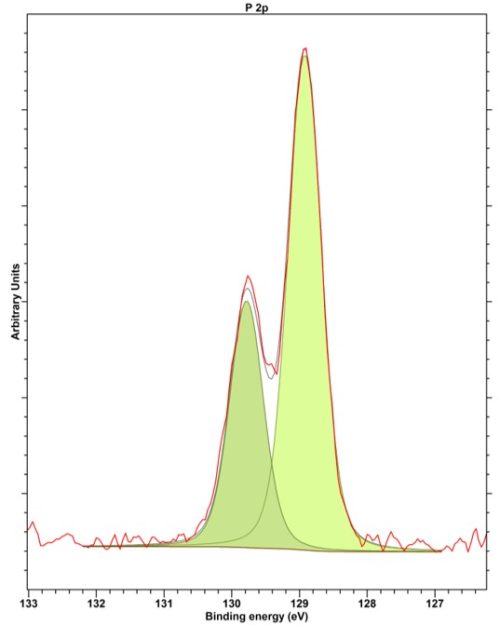
Figure 1: High resolution P 2p XP spectra from GaP showing the 0.86 eV splitting(1)
Phosphorous is commonly analysed in the context of bioglasses by XPS, where loss features, or high backgrounds from the Si 2p region may be seen. Ensure enough of a spectrum is taken at the low BE side to properly account for this Si contribution, and potentially use offsets to model the background.
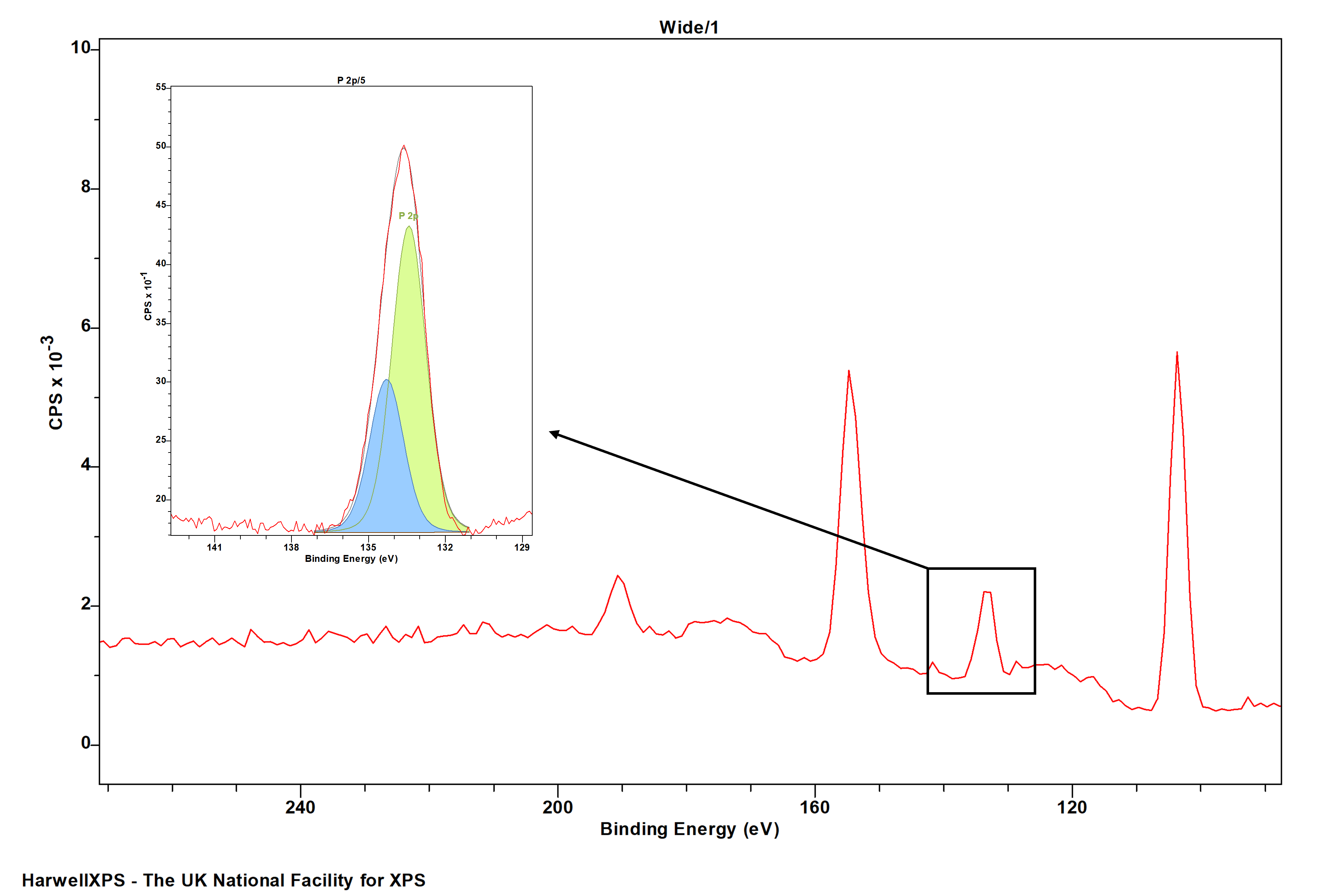
Though phosphorous is a doublet peak, the small doublet separation means people often fit this as one species. While we would not recommend this in general, it is OK for basic quantification, and providing the peak shape is kept consistent across different species.
Not available
Not available
References
- Data acquired by HarwellXPS
- Edmonds, M. T., et al. (2015). “Creating a stable oxide at the surface of black phosphorus.” ACS applied materials & interfaces 7(27): 14557-14562. Read it online here.
- Wang, Y. and P. M. Sherwood (2002). “Phosphorus pentoxide (P2O5) by XPS.” Surface Science Spectra 9(1): 159-165. Read it online here.
- Liu, K., et al. (2004). “Surface analysis of (NH2) 2CS‐treated GaP (001) by AES and XPS.” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 36(8): 966-968. Read it online here.