
Rhenium
Doublet Separations
- Re 4f: 2.4 eV
- Re 4d: 13.4 eV
- Re 4p: 71.9 eV
The Energies Listed are Binding Energies!
- Re 4s: 625 eV
- Re 4p: 445 eV
- Re 4d: 260 eV
- Re 4f: 40 eV
The Energies Listed are Binding Energies!
Re is primarily analysed via the 4f orbital
- V 3p (38 eV)
- Sr 4s (38 eV)
- Te 4d (40 eV)
- Ne 2s (41 eV)
- As 3d (41 eV)
- Tm MNN a (Al source) (43 eV)
- Cr 3p (43 eV)
- Ru 4p (43 eV)
- Ca 3s (44 eV)
- Y 4s (46 eV)
Energies listed are Kinetic Energies!
Re NOO: ~ 160 eV
The Energies Listed are Binding Energies!
| Material | Re(4f 7/2) Binding Energy / eV | Reference |
| Re Metal | 40.4 (Calibrated to Fermi Level) | [2] |
| Re2O7 | 46.8 (Calibrated to C(1s) at 284.8 eV) | [1, 2] |
| NH4ReO4 | 46.3 (Calibrated to C(1s) at 284.8 eV) | [2] |
| ReO2 | 43.0 (Std. Dev. of 0.6 eV) | [2] , ([3]) |
| ReO3 | 44.2 (Std. Dev of 1.3 ev) | [2] , ([3]) |
Typically considered to be one of the most stable elements due to its high melting and boiling points, rhenium is often found in nickel based superalloys, but also finds use in olefin metathesis and reforming catalysis. Rhenium can exhibit multiple oxidation states, with +7, +6, +4 and +2 the most common in materials analysed by XPS.
Figure 1, shows the spectra of a partially oxidised rhenium foil exhibiting metallic Re (green), ReO2 (blue) and Re2O7 (yellow) states.

High valence rhenium oxides can undergo disproportionation during XPS analysis [1], so a suitable methodology, such as rapid acquisition and recording the Re(4f) region first and again at the end should be considered.
Sb metal exhibits a modicum of asymmetry, for example – the clean Sb metal surface below may be fit with an LF(0.65,0.69,60,100,10) lineshape with the appropriate background. Important to note also, is the shake-up structure appearing at ~544 eV. The data below is available online via work from HarwellXPS technical director Dr. David Morgan at Surface Science Spectra.
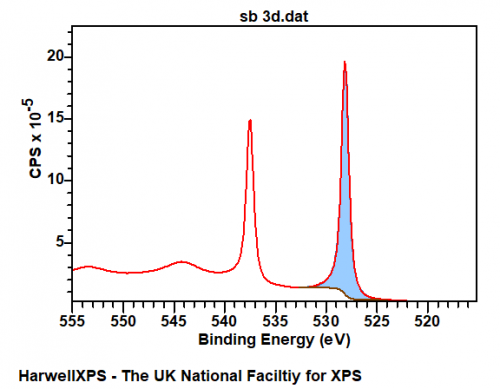
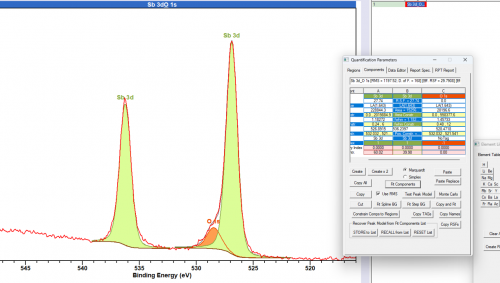
Fitting of the overlapping (with O 1s) Sb 3d 5/2 peak is made easier through using the unaffected 3d 3/2 peak. Peak constraints may be used to fix the overlapping peak to the ‘free’ peak, particularly important in the case of multiple Sb states.
Not available
Not available
References
[1] S. Iqbal, M. L. Shozi and D. J. Morgan, X‐ray induced reduction of rhenium salts and supported oxide catalysts, Surf. Inter. Anal. 49 (2017) 223-226. Read it online here
[2] Measured at HarwellXPS on a Thermo K-Alpha+ spectrometer, which has been calibrated using the internal ISO calibration method.
[3] Average of measurements from NIST. NIST Standard Reference Database 20, Version 4.1, Visit the site
