
Tellurium
Doublet Separations
- Te 3d: 10.4 eV
- Te 3p: 50.8 eV
- Te 4d: 1.45 eV
The Energies Listed are Binding Energies!
- Te 3s: 1006 eV
- Te 3p: 819 eV
- Te 3d: 572 eV
- Te 4s: 168 eV
- Te 4p: 110 eV
- Te 4d: 40 eV
The Energies Listed are Binding Energies!
Sb is primarily analyzed via the 3d orbital
- Cu MNN (Al source) (567 eV)
- Rn 4d (567 eV)
- Ag 3p (571 eV)
- Hg 4p (571 eV)
- Cr 2p (575 eV)
- Fr 4d (577 eV)
- Ir 4p (577 eV)
- Ru 3s (585 eV)
- W 4s (595 eV)

Energies listed are Kinetic Energies!
Te MNN: ~ 479 eV
The Energies Listed are Binding Energies!
| Species | Binding Energy / eV | Energy ref. / eV | Ref. |
| Te | 573.0 | Spectrometer Fermi level | 1 |
| Te | 573.1 | 84 (Au) | 2 |
| TeO2 | 576.1 | 284.4 (C) | 2 |
| TeO3 | 577.3 | 284.4 (C) | 2 |
| Te(OH)6 | 567.7 | 284.4 (C) | 2 |
| p-tolyl-TeOOH | 575.7 | 284.4 (C) | 2 |
| TeCl4 | 576.9 | 284.4 (C) | 2 |
| TeCI2 | 575.8 | 284.4 (C) | 2 |
| p-Methoxy-phenyl-TeCl3 | 576.3 | 284.4 (C) | 2 |
| (NH4)2TeCl6 | 576.5 | 284.4 (C) | 2 |
| TeBr4 | 576.7 | 284.4 (C) | 2 |
| Tel4 | 575.9 | 284.4 (C) | 2 |
| InTe | 572.7 | 84 (Au) | 4 |
| GaTe | 573 | 84 (Au) | 4 |
| In2Te3 | 572.5 | 84 (Au) | 5 |
| Ga2Te3 | 572.4 | 284.5 (C) | 6 |
| CdTe | 572.1 | 284.75 (C) | 7 |
| ZnTe | 573.1 | 285 (C) | 8 |
| Na2Te | 572.4 | 285 (C) | 8 |
| K2TeO3 | 575.7 | 285 (C) | 8 |
| (NH4)2TeO4 | 576.7 | 285 (C) | 8 |
| PbTe | 572.1 | 285 (C) | 9 |
| MnTe | 572.9 | 284.8 (C) | 10 |
Tellurium is a versatile element with several important uses. It is added to alloys, such as stainless steel and copper, to improve their machinability and workability. In the electronics industry, tellurium is used in semiconductors and photovoltaic cells, which are essential components of solar panels. Additionally, it acts as a coloring agent in glass and ceramics and is used in vulcanizing rubber. In battery technology, tellurium enhances the strength and hardness of lead, making it more resistant to the corrosive effects of acid in batteries.
XPS of tellerium is typically performed on the 3d orbitals. Potential overlaps include Ag (3p), Hg (4p), Cr (2p) and Ir (4p) though depending on the oxidation states involved, the overlap with Cr and Ir may be restricted to the Te 3d3/2 peak. The may also be an interference with the Cu LMM auger region, which is crucial to determining copper speciation. The doublet separation of Te 3d is 10.39 eV.1,2
Tellerium metal exists as a simple, well separated, doublet with a small degree of asymmetry, well separated from the most stable oxide form (TeO2).3
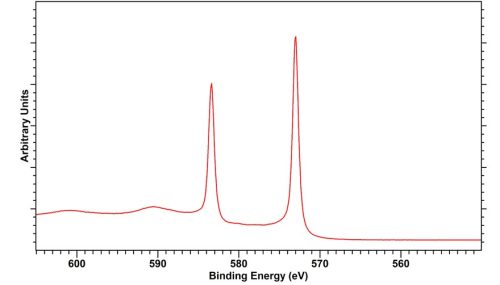
Tellurium Dioxide, TeO2
TeO2 isprone to x-ray induced reduciton. Whilst in our experience at HarwellXPS that this is slow for a bulk powder or thick oxide film. Those analysing thin films should take care and look to potentially minimise acquisition times to avoid analysis induced effects.
Chalcogenides
Te finds itself commonly used in it’s solid state chalcogenide form (ex. GaTe, InTe etc.) in applications relating to energy materials and thus, has been analysed thoroughly in these forms by XPS.
Te 3d binding energies are typically found below that of elemental Te, due to electronegativity differences (Te = 2.1, typical cations such as Zn, Cd, In = 1.6-1.8).*
*Pauling scale
Not only does the metal exhibit peak asymmetry in the Te 3d region, but also Te chalcogenides may produce a similar effect. Take care to ensure correct lineshape application in this region.

Additionally, take care when analysing the Te MNN auger, since this overlaps with the Te 3s signal and may cause erroneous calculations if determining auger parameter.
Not available
Not available
- Data recorded at HarwellXPS
- Bahl, M., et al. (1977). “X‐ray photoemission studies of tellurium and some of its compounds.” The Journal of chemical physics 66(12): 5526-5535. Read it online here.
- Musket, R. (1978). “Studies of clean and oxidized tellurium surfaces.” Surface science 74(2): 423-435. Read it online here.
- Balitskii, O. and W. Jaegermann (2006). “XPS study of InTe and GaTe single crystals oxidation.” Materials chemistry and physics 97(1): 98-101. Read it online here.
- Guettari, N., et al. (2003). “InxTey semiconductor thin films obtained by co-evaporation.” Thin Solid Films 431: 497-501. Read it online here.
- Gillan, E. G. and A. R. Barron (1997). “Chemical vapor deposition of hexagonal gallium selenide and telluride films from cubane precursors: Understanding the envelope of molecular control.” Chemistry of Materials 9(12): 3037-3048. Read it online here.
- Poirier, D. and J. Weaver (1993). “CdTe (110) by XPS.” Surface Science Spectra 2(3): 209-216. Read it online here.
- Sartz, W. E., et al. (1971). “X-ray photoelectron spectroscopic investigation of Group VIA elements.” Analytical Chemistry 43(13): 1884-1887. Read it online here.
- Yashina, L., et al. (2004). “The oxidation of PbTe (100) surface in dry oxygen.” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 36(8): 993-996. Read it online here.
- Iwanowski, R., et al. (2005). “Sputter cleaning and annealing of zinc-blende MnTe surface—XPS study.” Applied Surface Science 249(1-4): 222-230. Read it online here.