- Energy Dependency: The transmission function is usually energy-dependent. It means that it may vary with the kinetic energy of the emitted photoelectron. Different components of the spectrometer, such as the entrance slit, electrostatic lens, and various apertures, can affect the transmission function differently at different energies.
- Instrument-Dependent: The transmission function is specific to the design and configuration of the XPS instrument being used. It depends on factors like the geometry of the instrument, the quality of various optical elements, and the calibration of the instrument.
- Normalization: To analyze XPS spectra quantitatively, it is essential to consider the transmission function when calculating peak intensities or determining atomic concentrations. Without this correction, the intensities in the XPS spectra may not accurately represent the actual number of photoelectrons emitted from the sample.
- Measurement and Calibration: Accurate determination of the transmission function usually requires careful calibration of the XPS instrument. This may involve measuring the transmission function using well-characterized reference samples with known photoelectron emission characteristics.
- Data Analysis: Modern XPS data analysis software typically applied transmission function corrections automatically – so there is no need for the analyst to concern themselves with it. This correction accounts for the instrument’s efficiency and allows for more accurate quantification of elemental concentrations and chemical states in the sample.
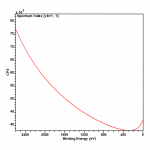
Transmission Function
In X-ray Photoelectron Spectroscopy (XPS), the term “transmission function” typically refers to the efficiency or transmission probability of the spectrometer for detecting photoelectrons. It is an important parameter to consider when analysing XPS spectra as it affects the accuracy and sensitivity of the measurements.
Transmission function needs to be properly accounted for if one wishes to accurately quantify the signal intensity from an XPS experiment.
Different instruments use different quantification methods (e.g. Schofield factors, modified Wagner factors etc).