
Antimony
Doublet Separations
- Zn 2p: 23.1 eV
- Zn 3p: 2.7 eV
- Zn 3d: 0.7 eV
The Energies Listed are Binding Energies!
- Zn 2s: 1191 eV
- Zn 2p: 1020 eV
- Zn 3s: 140 eV
- Zn 3p: 87 eV
The Energies Listed are Binding Energies!
Zn is primarily analyzed via the 2p orbital
- V LMM (Al source) (1018 eV)
- Pm 3d (1027 eV)
- Sb MNN (Al source) (1032 eV)
- At 4s (1042 eV)
- U 4s (1043 eV)
Energies listed are Kinetic Energies!
Zn MNN: ~ 991 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref. | Ref. |
| Zn | 1021.65 | Au 4f / 83.95 eV | 1 |
| ZnO | 1021.7 | C 1s / 285 eV | 2 |
Table 1: Zinc binding energies
Zinc exhibits minimal differences in the binding energy of different chemical states, due to a number of factors:
Zinc analysis is typically performed on the Zn 2p photoemission, however obtaining the Zn KLL auger region is often paramount for interpreting the data correctly. There may be a slight overlap between the Zn 2p peaks and the O and V KLL augers from Al X-rays, however it should not impede deconvolution, partly due to the large doublet separation (23 eV).
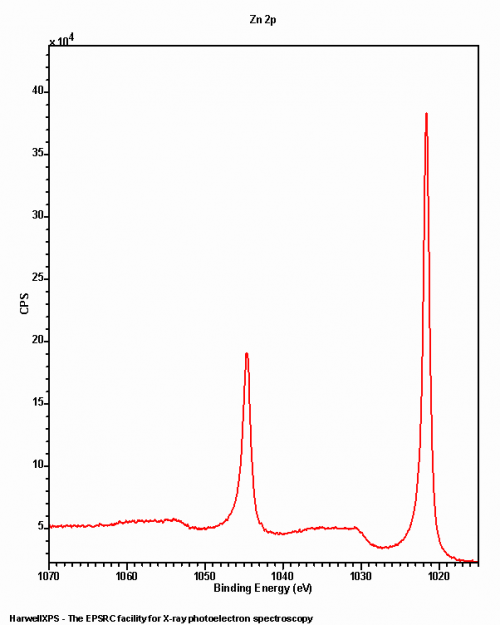
Common binding energies for Zinc species may be found below:
| Species | Binding energy / eV | Charge Ref. | Ref. |
| Zn | 1021.65 | Au 4f / 83.95 eV | 1 |
| ZnO | 1021.7 | C 1s / 285 eV | 2 |
While the separation above may be small, as mentioned previously, the Zn LMM auger may assist in spectral understanding via the modified Auger parameters (table 2).

Not available
Not available
- Data acquired by HarwellXPS
- Ramgir, N. S., et al. (2006). “ZnO multipods, submicron wires, and spherical structures and their unique field emission behavior.” The Journal of Physical Chemistry B 110(37): 18236-18242. Read it online here.
- Diler, E., et al. (2014). “Initial formation of corrosion products on pure zinc and MgZn2 examinated by XPS.” Corrosion science 79: 83-88. Read it online here.
- Leontiev, S. A., et al. “Detailed XPS and UPS studies of the band structure of zinc oxide.” Journal of structural chemistry 38 (1997): 725-731. Read it online here.