
Ruthenium
Doublet Separations
- Ru 3d: 4.2 eV
- Ru 3p: 22.4 eV
- Ru 4p: 3 eV
The Energies Listed are Binding Energies!
- Ru 3s: 585 eV
- Ru 3p: 461 eV
- Ru 3d: 284 eV
- Ru 4s: 75 eV
- Ru 4p: 43 eV
The Energies Listed are Binding Energies!
Ru is primarily analysed via the 3d orbital
The major consideration for analysis of Ru is the significant overlap with the C 1s peak – an ever present in XPS analysis!

Other potential overlaps:
- Os 4d (273 eV)
- Re 4d (274 eV)
- Fr 4f (276 eV)
- Er MNN a (Al source) (277 eV)
- Sr 3p (280 eV)
- Eu 4p (284 eV)
- C 1s (285 eV)
- Tb 4p (286 eV)
- Kr 3s (289 eV)
- Gd 4p (289 eV)
- Os 4d (290 eV)
- Ce 4s (290 eV)
- K 2p (293 eV)
- Dy 4p (293 eV)
- Ir 4d (295 eV)

Energies listed are Kinetic Energies!
Ru MNN: ~ 270 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| Ru(0) | 280 | ||
Ruthenium (Ru) is a metal typically found in catalysis, electrochemistry and electrical applications.
XPS analysis of ruthenium is complicated due to the main Ru(3d) core-level overlapping with the C(1s) region, so spectra can be dominated by adventitious carbon signals, or that of an activated carbon support.
As with many metals, Ru exhibits asymmetry to its metallic core-lines, with RuO2 also exhibiting asymmetric Ru(3d) and also O(1s) core levels due to the conducting nature of the material (as shown below).
Ru 3d 5/2 and 3/2 peaks have differing FWHM due to Coster-Kronig effects.
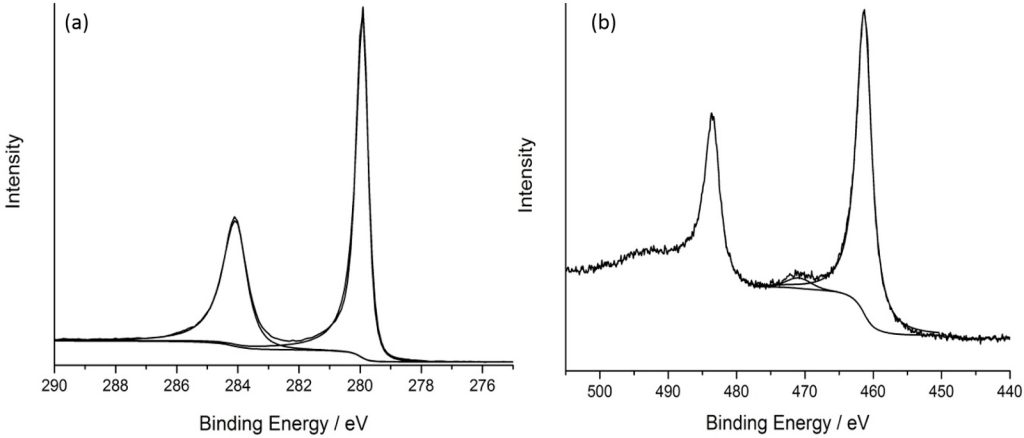
RuO2 is found typically in one of two forms; anhydrous and the more common hydrated form which clearly shows the asymmetric nature of the peaks and screened states giving rise to satellite species often confused with higher oxidation states and can be seen below, together with the adventitious carbon overlap.

RuCl3 is known to reduce under X-ray illumination, and should be analysed with care (see dealing with sample damage)
Not available
Not available