- Elements
Silicon
Silicon is extensively studied by XPS due it’s effectiveness as a substrate and the versatility of the oxide as a catalyst support. The 2p region is the major emission where there is a small doublet separation (0.6 eV) and the oxidised form of silicon may exhibit a larger FWHM than that of the elemental form (Figure 1).
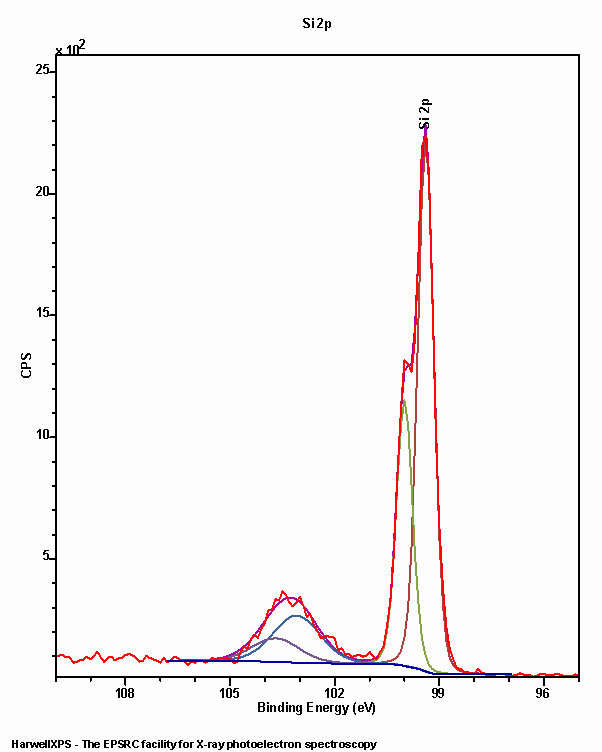
Silicon species exhibit marked differences in binding energy, rendering deconvolution a relatively trivial task. A range of common binding energies may be found in Table 1.
Silicon 2s peaks are also relatively large, and may be of use where Si 2p overlaps with another emission (Figure 2).

References
- Data acquired by HarwellXPS
- Jensen, D. S., et al. (2013). “Silicon (100)/SiO2 by XPS.” Surface Science Spectra 20(1): 36-42. Read it online here.
- Miyoshi, K. and D. H. Buckley (1982). “XPS, AES and friction studies of single-crystal silicon carbide.” Applications of Surface Science 10(3): 357-376.. Read it online here.

Antimony
Doublet Separations
- Si 2p: 0.6 eV
The Energies Listed are Binding Energies!
- Si 2p: 99 eV
- Si 2s: 150 eV
- Si 1s: 1840 eV (HAXPES only)
The Energies Listed are Binding Energies!
Si is primarily analysed via the 2p orbital. Given the low energy, there are many potential overlaps. The most significant are outlined below.
- Fe 3s (95 eV)
- Ag 4s (95 eV)
- Al KLL (Al source) (97 eV)
- Co 3s (103 eV)
 The full list is as follows:
The full list is as follows:
- Bi 5p (93 eV)
- Ba 4d (93 eV)
- Fe 3s (95 eV)
- Ag 4s (95 eV)
- Th 5d (95 eV)
- U 5d (96 eV)
- Ir 5s (96 eV)
- Br LMM (Al source) (97 eV)
- Al KLL (Al source) (97 eV)
- La 4d (99 eV)
- Sb 4p (99 eV)
- Hg 4f (99 eV)
- Tl 5p (100 eV)
- Co 3s (101 eV)
- Pt 5s (102 eV)
- Ga 3p (103 eV)
- Po 5p (104 eV)
- U 5d (104 eV)
- Pb 5p (105 eV)
- Cd 4s (108 eV)
- Au 5s (108 eV)

Energies listed are Kinetic Energies!
Si LMM: ~ 93 eV
Si KLL: ~ 1610 eV (HAXPES only)
The Energies Listed are Binding Energies!
Silicon species exhibit marked differences in binding energy, rendering deconvolution a relatively trivial task. A range of common binding energies may be found in Table 1.
Silicon is extensively studied by XPS due it’s effectiveness as a substrate and the versatility of the oxide as a catalyst support. The 2p region is the major emission where there is a small doublet separation (0.6 eV) and the oxidised form of silicon may exhibit a larger FWHM than that of the elemental form (Figure 1).

Silicon 2s peaks are also relatively large, and may be of use where Si 2p overlaps with another emission (Figure 2).

Antimony systems are not known to interact in any significant way with experimental conditions during XPS analysis.
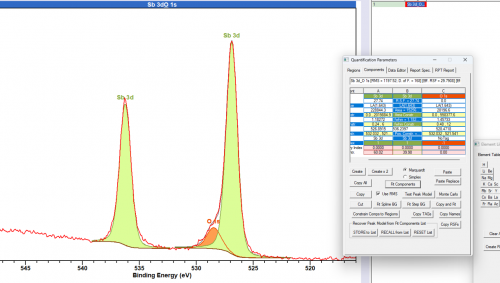
Care should be taken to record both the 3d 5/2 and the 3d 3/2 peaks when obtaining data for antimony based systems, since the 3/2 emissions will be vital in proper data handling of the 5/2 emission due to overlap with the O 1s feature.
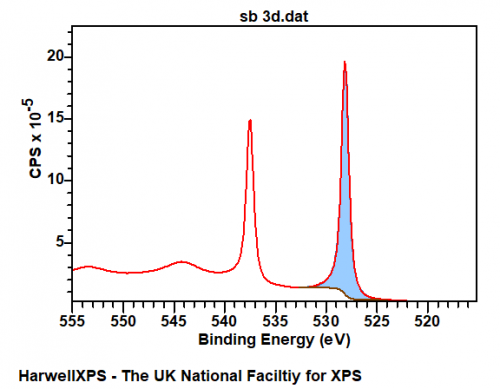
Sb metal exhibits a modicum of asymmetry, for example – the clean Sb metal surface below may be fit with an LF(0.65,0.69,60,100,10) lineshape with the appropriate background. Important to note also, is the shake-up structure appearing at ~544 eV. The data below is available online via work from HarwellXPS technical director Dr. David Morgan at Surface Science Spectra.
Fitting of the overlapping (with O 1s) Sb 3d 5/2 peak is made easier through using the unaffected 3d 3/2 peak. Peak constraints may be used to fix the overlapping peak to the ‘free’ peak, particularly important in the case of multiple Sb states.
Not available
Not available
- Data acquired by HarwellXPS
- Jensen, D. S., et al. (2013). “Silicon (100)/SiO2 by XPS.” Surface Science Spectra 20(1): 36-42. Read it online here.
- Miyoshi, K. and D. H. Buckley (1982). “XPS, AES and friction studies of single-crystal silicon carbide.” Applications of Surface Science 10(3): 357-376.. Read it online here.