
Sulfur
Doublet Separations
- S 2p: 1.2 eV
The Energies Listed are Binding Energies!
- S 2p: 165 eV
- S 2s: 225 eV
- S 1s: 2480 eV (HAXPES only)
The Energies Listed are Binding Energies! Sb is primarily analyzed via the 3d orbital
- Se 3p (162 eV)
- Cs 4p (162 eV)
- Bi 4f (163 eV)
- Rn 5p (164 eV)
- Ac 5p (167 eV)
- Te 4s (168 eV)
- Er 4d (168 eV)

Energies listed are Kinetic Energies! Sb MNN: ~ 450 eV
The Energies Listed are Binding Energies!
Typical binding energies for sulfur compounds may be found in table 1.
| Species | Si 2p3/2 Binding energy / eV | Charge ref | Ref |
| S8 | 164.3 | N/A | 2 |
| Li2S | 160.8 | N/A | 3 |
| Thiol, R-SH | 162 | C 1s / 284.6 eV | 4 |
| Thiol, Au-SH | 162.8 | Au 4f / 84 eV | 5 |
| Sulfonic acid (R-SO3H) | ~168 | C 1s / 284.8 eV | 6 |
| Sulfated zirconia | 169.2 | C 1s / 284.6 eV | 7 |
| CuSO4 | 168.8 | C 1s / 284.6 eV | 8 |
The predominant photoemission for sulfur is the 2p region, which consists of a doublet with a reasonable doublet separation (1.16 eV – Figure 1). This region may overlap with Bi 4f and Se 3p.
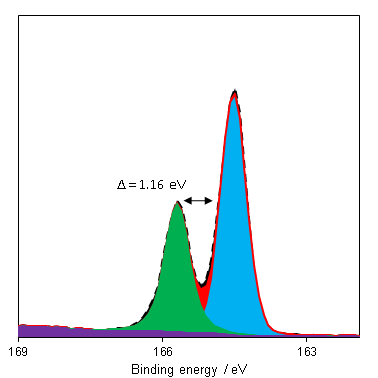
Since binding energy shifts may be small for organic sulfurs and polysulfides, the S 2p:KLL auger parameter may make state identification simpler.(1) Si 2s plasmons can overlap with the S 2p region and render appropriate background simulation difficult in the cases of low S:Si ratio. Recording an extended background, for both Si 2s and Si 2p, can help properly assess the rising background post Si peak.
Si 2s plasmons can overlap with the S 2p region and render appropriate background simulation difficult in the cases of low S:Si ratio. Often an extrapolated Shirley, or even a quadratic background (If [Si] >>> [S]), can help correctly assess the rising Si emission.
Not available
Not available
- Spectra recorded by HarwellXPS
- Fantauzzi, M., et al. (2014). “A contribution to the surface characterization of alkali metal sulfates.” Journal of Electron Spectroscopy and Related Phenomena 193: 6-15. Read it online here.
- Fantauzzi, M., et al. (2015). “Exploiting XPS for the identification of sulfides and polysulfides.” RSC advances 5(93): 75953-75963. Read it online here.
- Wilson, K., et al. (2002). “Structure and reactivity of sol–gel sulphonic acid silicas.” Applied Catalysis A: General 228(1-2): 127-133. Read it online here.
- Sun, S., et al. (2006). “Fabrication of gold micro-and nanostructures by photolithographic exposure of thiol-stabilized gold nanoparticles.” Nano letters 6(3): 345-350. Read it online here.
- Isaacs, M. A., et al. (2019). “Unravelling mass transport in hierarchically porous catalysts.” Journal of Materials Chemistry A 7(19): 11814-11825. Read it online here.
- Rabee, A. I., et al. (2017). “Acidity-reactivity relationships in catalytic esterification over ammonium sulfate-derived sulfated zirconia.” Catalysts 7(7): 204. Read it online here.
- Vasquez, R. (1998). “CuSO4 by XPS.” Surface Science Spectra 5(4): 279-284. Read it online here.